Improving Heart Transplantation by Expanding the Donor Pool
Quick Takes
- The TransMedics™ Organ Care System (OCS) Heart is a portable system for extracorporeal warm perfusion and assessment of donated hearts.
- FDA approval of the OCS is expected for the preservation of both extended criteria donor hearts and hearts with expected prolonged time between procurement and transplant.
- Ex vivo perfusion technologies such as the OCS may soon be applied to donation after circulatory death heart transplant, a paradigm with the potential to significantly expand the donor pool.
Introduction
Despite advancements in medical therapy and mechanical circulatory support, heart transplantation remains the optimal treatment for end-stage heart failure in terms of survival and quality of life.1,2 While the number of heart transplants performed in the United States continues to increase, so does the number of heart transplant candidates. In 2018, for example, over 3,900 adult patients were newly listed for heart transplant, but only 2,940 adult heart transplants were performed, and 540 patients were removed from the waiting list due to death or clinical deterioration.3 The increasing demand for donor hearts has catalyzed various efforts to expand their supply and utilization rate.
Here we describe recent advancements in, and implications of, one such effort: the use of ex vivo perfusion technology, specifically the TransMedics™ OCS, for extracorporeal heart preservation and assessment. This system allows for transportation of a donor heart while still beating and metabolically active, as opposed to traditional transportation via cold storage on ice. In April 2021, a Food and Drug Administration (FDA) panel voted in favor of approval of the OCS for the preservation of both extended criteria donor hearts and hearts with expected prolonged cross-clamp time.4 We describe the primary study on which this decision was based (OCS Heart EXPAND), an ongoing study involving use of the OCS for preservation of hearts donated after circulatory death (DCD Heart), and implications of each for the field of heart transplantation.
TransMedics™ OCS Heart
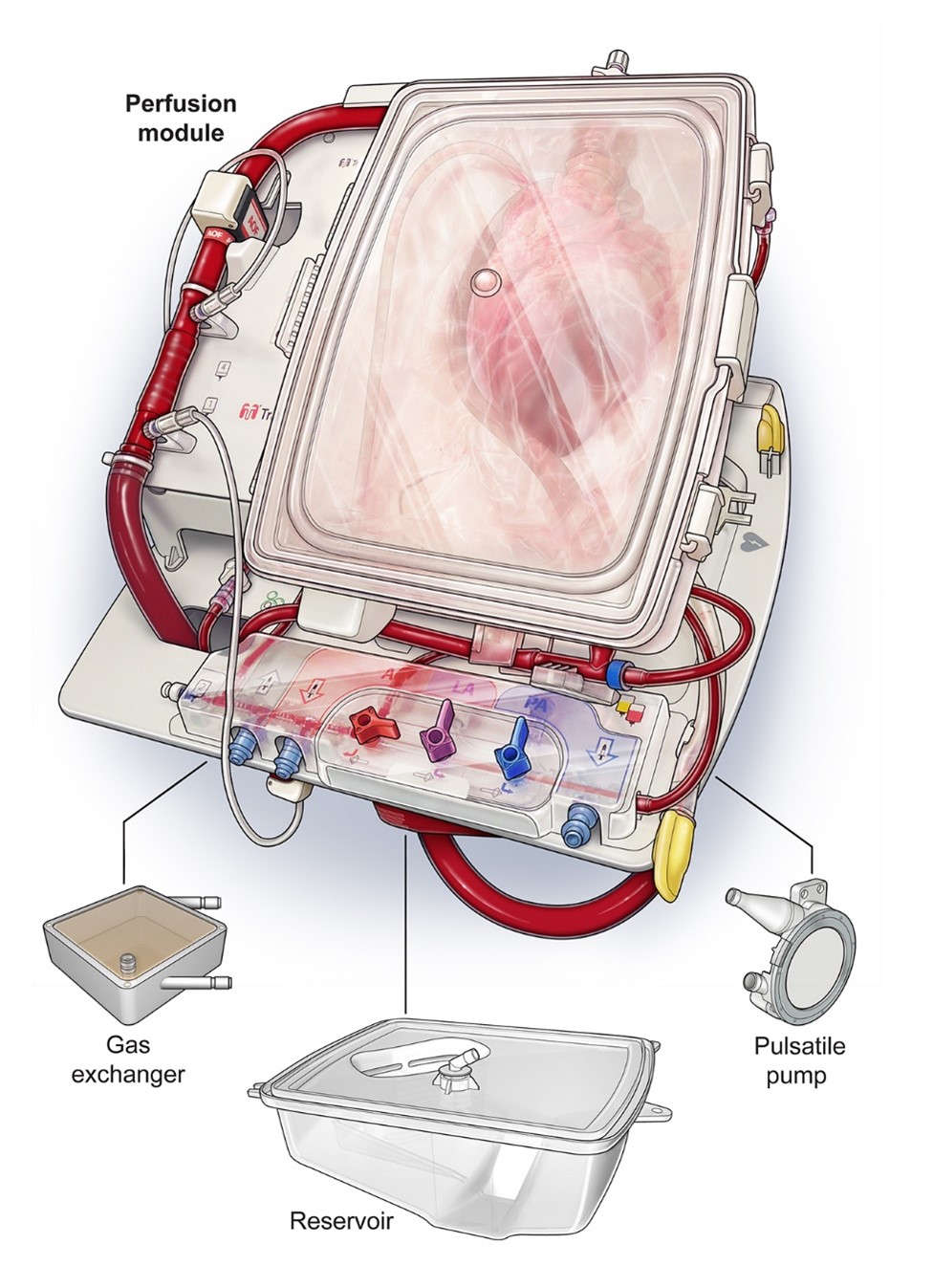
The TransMedics™ OCS Heart is a portable system for extracorporeal heart perfusion, assessment, and resuscitation. It preserves the heart in a beating state via retrograde perfusion, wherein oxygenated donor blood enters the coronary arteries through the aorta and returns to the circuit through the pulmonary artery (Figure 1). Functional heart assessment is possible via visual assessment and monitoring of circulating lactate levels and perfusion pressures.
Figure 1: TransMedics™ OCS Heart
OCS Heart EXPAND Trial
Background: The suitability and transportability of donor hearts is currently limited by cold ischemic time, the amount of time the organ is preserved outside of the body on ice. Marginal donor hearts, such as those from extended criteria donors, are particularly prone to physiologic insult from prolonged cold ischemia. The OCS limits cold ischemia via extracorporeal normothermic perfusion, thus potentially allowing both the expanded utilization of extended criteria donor hearts and the distribution of hearts across broader geographic areas.
Design & Cohort: OCS Heart EXPAND was a prospective, single-arm, multi-center trial to evaluate the use of the OCS to preserve and assess extended criteria donor hearts for transplantation.6 Donor hearts meeting either of the following characteristics were eligible: 1) expected total ischemic time ≥4 hours, or 2) expected total ischemic time ≥2 hours and at least one of the following risk factors: left ventricular hypertrophy, EF 40-50%, downtime ≥20 minutes, donor age >55 years. Included were 93 eligible donor hearts with a mean UNOS match run of 66 declines.
Results:
- 75 of 93 extended criteria donor hearts perfused on the OCS were transplanted (81% utilization rate)
- Mean cross-clamp time and OCS perfusion time were 381 minutes and 279 minutes, respectively
- 30-day survival was 95% (vs. UNOS national average for standard criteria donation of 96%)
- Incidence of severe primary graft dysfunction within 24 hours of transplant was 11%
- Overall and cardiac-related survival at 24 months were 82% and 95%, respectively
Implications: The OCS permits the successful transplantation of extended criteria donor hearts not routinely transplanted today due to an increased risk of primary graft dysfunction. It is thus a valuable tool in the effort to expand the donor pool via the widespread utilization of extended criteria donor hearts. Despite prolonged time between organ procurement and transplant, outcomes among recipients of OCS-perfused hearts were comparable to those of standard criteria donor heart recipients, demonstrating the potential utility of the OCS to expand the feasible geographic distance between heart procurement and transplantation.
DCD Heart Trial
Background: An emerging strategy to expand the donor pool is the use of donation after circulatory death (DCD) as a supplement to donation after brain death (DBD), which currently comprises the vast majority of heart donation. In contrast to DBD donors, who meet full criteria for brain death prior to organ procurement, DCD donors have suffered devastating and irreversible brain injury but do not meet criteria for brain death, and thus require withdrawal of life-sustaining therapy before a pronouncement of circulatory death can be made. Unlike DBD hearts, which are continuously perfused until procurement, DCD hearts are subject to a phase of warm ischemia, defined as the period of hypoperfusion between withdrawal of life-sustaining therapy and organ reperfusion. Recent advances in ex vivo perfusion have allowed the assessment and resuscitation of DCD hearts and contributed to an expansion of DCD, which has the potential to increase the US donor pool by 30% and substantially decrease waiting list mortality.7
Design & Cohort: DCD Heart is an ongoing, prospective, multi-center, non-inferiority trial comparing the transplantation of DCD hearts resuscitated on the OCS to transplantation with standard criteria DBD hearts preserved using traditional cold storage methods.8 Eligible DCD donors were those age 18-49 years meeting Maastricht Category III criteria (expected death after withdrawal of life-sustaining therapy), with warm ischemic time ≤30 minutes, and without any of the following: previous cardiac surgery, known coronary artery disease, cardiogenic shock, myocardial infarction, EF ≤50%, or significant valve disease.
Implications: Enrollment in the trial has gone well and the results should be available soon. Ex vivo perfusion may play a role in the expansion of DCD transplantation. It is important to note, however, that DCD transplantation is possible without ex vivo perfusion through other techniques including normothermic regional perfusion, in which the heart is resuscitated in situ and in isolation within the donor via extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass configured to selectively perfuse parts of the body but not the brain.
Future Directions
Ex vivo perfusion technologies such as the OCS will expand the donor pool and increase the number of patients that can receive heart transplantation. The technologies are also promising for other areas of donor management that could improve heart transplantation. For example, ex vivo perfusion may afford an opportunity to administer therapies to the donor heart prior to transplantation to improve the metabolic state of the heart after an insult such as recent cardiac arrest, or to modify the immunologic state of the heart and minimize rejection in the recipient. These are active areas of research.
References
- Wilhelm MJ. Long-term outcome following heart transplantation: current perspective. J Thorac Dis 2015;7:549-51.
- Mullan CW, Sen S, Ahmad T. Left ventricular assist devices versus heart transplantation for end stage heart failure is a misleading equivalency. JACC Heart Fail 2021;9:290-92.
- Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2018 Annual Data Report: Heart. Am J Transplant 2020;20:340-426.
- Draft Summary of Safety and Efficacy Data (SSED) (fda.gov). 2021. Available at: https://www.fda.gov/media/147297/download. Accessed 05/15/2021.
- Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577-84.
- Schroder JN, D'Alessandro D, Esmailian F, et al. Successful utilization of extended criteria donor (ECD) hearts for transplantation – results of the OCS Heart EXPAND trial to evaluate the effectiveness and safety of the OCS Heart System to preserve and assess ECD Hearts for transplantation. J Heart Lung Transplant 2019;38:S42.
- Jawitz OK, Raman V, DeVore AD, et al. Increasing the United States heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg 2020;159:e307-e309.
- Shudo Y, Benjamin-Addy R, Koyano TK, et al. Donors after circulatory death heart trial. Future Cardiol 2021;17:11-17.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Implantable Devices, SCD/Ventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Acute Heart Failure, Heart Transplant, Interventions and Coronary Artery Disease, Interventions and Vascular Medicine
Keywords: Heart Failure, Cold Ischemia, Warm Ischemia, Heart Transplantation, Extracorporeal Membrane Oxygenation, Quality of Life, Waiting Lists, Primary Graft Dysfunction, Cardiopulmonary Bypass, Shock, Cardiogenic, Coronary Artery Disease, Pulmonary Artery, Hypertrophy, Left Ventricular, United States Food and Drug Administration, Prospective Studies, Tissue Donors, Tissue and Organ Procurement, Perfusion, Myocardial Infarction, Heart Arrest, Reperfusion, Aorta, Risk Factors, Brain Injuries, Brain, Technology, Catalysis, Withholding Treatment, Reference Standards, Lactates
< Back to Listings