A Novel Therapeutic Approach for the Treatment of PAH: Results From PULSAR
Quick Takes
- In PULSAR (A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension), a phase 2 clinical trial, sotatercept significantly reduced pulmonary vascular resistance (PVR) compared to placebo in patients with pulmonary arterial hypertension (PAH).
- Sotatercept represents a potential novel treatment option for patients with PAH and may lead to vascular wall remodeling.
- Phase 3 clinical trials with sotatercept are ongoing.
The pathophysiology of PAH is characterized by a loss of pulmonary vascular bed leading to a rise in PVR. One of the phenomena involved is endothelial dysfunction, a process resulting in an imbalance of vasomotor function favoring vasoconstriction over vasodilation due to underproduction of vasodilator mediators (nitric oxide and prostacyclin) and overproduction of vasoconstrictor agents (endothelin-1). Added to this phenomenon, vascular wall remodeling occurs and is characterized by proliferation of the pulmonary vascular smooth muscle cells (VSMC) with structural changes to the pulmonary arterial wall.
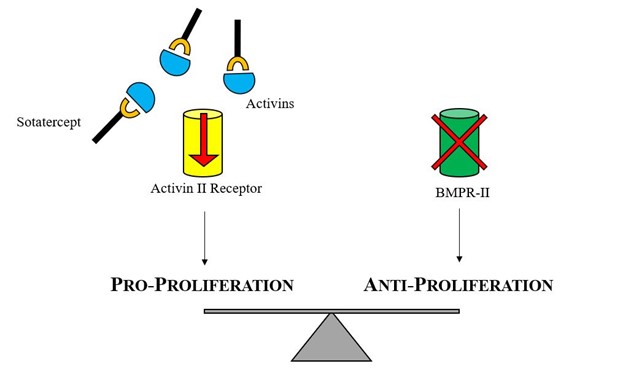
BMPR2 is a serine/threonine receptor kinase that binds to proteins, is a member of the transforming growth factor beta (TGF-beta) superfamily, and is involved in regulating cell growth and differentiation, among other functions. Activation of this receptor inhibits the proliferation of VSMC by promoting survival of the endothelial cells, thereby preventing arterial wall damage. Mutations related with PAH produce a loss of function of this receptor and, in consequence, promote VSMC proliferation and arterial wall remodeling. It has been long recognized that over two thirds of cases of hereditable PAH and a smaller proportion of idiopathic PAH cases involve a mutation in BMPR2.
Currently, several treatment options exist to manage PAH that are largely targeted to restore the vasoconstriction/vasodilation balance. Existing PAH-specific medical therapy currently targets the endothelin, nitric oxide, and prostacyclin pathways. Despite the available treatment options, 5-year survival in PAH is estimated to be 57% based on registry data. During the 6th World Symposium for Pulmonary Hypertension, an urgent need to identify new therapy specifically targeting pulmonary vascular remodeling and inflammation was highlighted.
Sotatercept is a novel fusion protein containing the extracellular domain of the human activin receptor type IIA and the Fc domain of human immunoglobulin G1, which acts as a ligand trap. Sotatercept binds to ligands in the TGF-beta superfamily (activin A and B, growth differentiation factors 8 and 11), which are associated with promoting cell proliferation and differentiation. By binding and "trapping" these ligands, sotatercept restores balance between the normally functioning growth-promoting cell pathway (activin IIA) and the impaired growth-inhibiting pathway (BMPR2).
PULSAR is a phase 2, double-blinded, randomized, placebo-controlled, multicenter, parallel-group study designed to determine the efficacy and safety of sotatercept versus placebo added to standard of care in patients with PAH. Subjects included in the study had a diagnosis of PAH (excluding subtypes related to HIV, schistosomiasis, and portal hypertension), World Health Organization functional class II-III, right heart catheterization with PVR >5 Wood units, and 6-minute walk distance between 150 and 550 m. Key exclusion criteria included uncontrolled systemic hypertension, baseline hemoglobin >16 g/dL, and platelets <100,000/mcL. Stable dose of background medical therapy with monotherapy or combination therapy endothelin antagonist, phosphodiesterase-5 inhibitors, soluble guanylate cyclase stimulators, and prostacyclin pathway agents was allowed. Subjects were randomly assigned in a 3:3:4 ratio to placebo, sotatercept 0.3 mg/kg, or sotatercept 0.7 mg/kg. Sotatercept was administered as a subcutaneous injection every 21 days.
A total of 106 patients was randomized and included in the analysis. Overall, most patients were white (92%) and female (87%) with a mean age of 48.3 years old. The majority had idiopathic PAH (58%) with a smaller percentage representing familial PAH (16%), PAH associated with connective-tissue disease (17%), drug-induced or toxin-induced PAH (7%), and PAH associated with corrected congenital shunts (3%). Background PAH-specific medical therapy included monotherapy (9%), double combination therapy (35%), and triple combination therapy (56%). A significant number of patients were receiving prostacyclin infusion therapy (37%). The average mean pulmonary artery pressure was 52.4 mmHg, cardiac index was 2.6 L/min/m2, and PVR was 9.7 Wood units (778.6 dynes·sec·cm-5).
In PULSAR, sotatercept significantly decreased PVR, which was the primary endpoint for the study. The least squares mean change from baseline in PVR was a decrease of 2 Wood units (162.2 dynes·sec·cm-5) in subjects in the sotatercept 0.3 mg group, a decrease of 3.2 Wood units (255.9 dynes·sec·cm-5) in the sotatercept 0.7 mg group, and a decrease of 0.2 Wood units (16.4 dynes·sec·cm-5) in the placebo group. These results were consistent across prespecified subgroups such as monotherapy versus combination therapy and prostacyclin use or not. Sotatercept also had significant improvement in the following secondary endpoints:
- 6-minute walk distance (an increase of 58.1 m in the sotatercept 0.3 mg group, increase of 50.1 m in the sotatercept 0.7 mg group, and increase of 28.7 m in the placebo group)
- N-terminal pro-B-type natriuretic peptide (decrease of 621.1 pg/ml in the sotatercept 0.3 mg group, decrease of 340.6 pg/ml in the sotatercept 0.7 mg group, and an increase of 310.4 pg/ml in the placebo group)
- World Health Organization functional class (improvement from baseline by at least 1 class in 12% in the placebo group, 31% in the sotatercept 0.3 mg group, and 17% in the sotatercept 0.7 mg group)
PULSAR brings to light a potential new treatment option for patients with PAH with a novel approach that may lead to pulmonary vascular wall remodeling. PULSAR included a patient population that was prevalent and treated with current standard of care, PAH-specific therapies including parenteral prostacyclins. The treatment effects were consistent across subgroups. The fact that the reduction of PVR was mainly driven by a reduction of mean pulmonary artery pressure without change in PVR or pulmonary capillary wedge pressure suggests the mechanism of action of sotatercept may be primarily vascular wall remodeling as opposed to vasodilatation. The drug was well tolerated. Thrombocytopenia was the most common adverse event of special interest, although there were no events of thrombocytopenia related bleeding, and no subjects required a platelet transfusion.
It is important to remember that PULSAR is a phase 2 clinical trial, and larger, long-term studies are needed to confirm and validate the efficacy of sotatercept. Currently, several phase 3 clinical trials are underway studying sotatercept in diverse clinical scenarios, such as PAH on background medical therapy, recently diagnosed PAH, PAH with high risk for mortality, and long-term follow-up studies. These studies will help clinicians better understand the impact that sotatercept may have in PAH.
Figure 1
References
- Humbert M, McLaughlin V, Gibbs JSR, et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med 2021;384:1204-15.
- Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019;53:1801887.
- Sitbon O, Gomberg-Maitland M, Granton J, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J 2019;53:1801908.
- Guignabert C, Humbert M. Targeting transforming growth factor-β receptors in pulmonary hypertension. Eur Respir J 2021;57:2002341.
Clinical Topics: Anticoagulation Management, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Lipid Metabolism, Heart Failure and Cardiac Biomarkers, Pulmonary Hypertension, Hypertension
Keywords: Endothelin-1, Epoprostenol, Vasodilation, Ligands, Nitric Oxide, Angiotensin-Converting Enzyme Inhibitors, DEAE-Dextran, Muscle, Smooth, Vascular, Phosphodiesterase 5 Inhibitors, Hypertension, Pulmonary, Pulmonary Wedge Pressure, Prostaglandins I, Vasodilator Agents, Platelet Transfusion, Natriuretic Peptide, Brain, Vasoconstriction, Vasoconstrictor Agents, Transforming Growth Factor beta, Endothelial Cells, Pulmonary Artery, Least-Squares Analysis, Blood Platelets, Follow-Up Studies, Follow-Up Studies, Standard of Care, Schistosomiasis, Schistosomiasis, Hemoglobins, Pharmaceutical Preparations, Immunoglobulins, Cell Proliferation, Thrombocytopenia, Cell Differentiation, Connective Tissue Diseases, Hypertension, Portal, Inflammation, Cardiac Catheterization, Registries, World Health Organization, Injections, Subcutaneous, Growth Differentiation Factors, Growth Differentiation Factors, Endothelin Receptor Antagonists, Threonine, Serine, Activins
< Back to Listings