Obesity in Patients with Advanced Heart Failure and Left Ventricular Assist Devices
Obesity and Heart Failure
Excess adiposity is a clear risk factor for subsequent heart failure (HF) development, with the strongest dose-response relationship between body mass index (BMI) and incident HF being for heart failure with preserved ejection fraction (HFpEF).1 Successful weight loss, for example with bariatric surgery, can reduce incident HF by more than half.2 Optimal management of obesity for patients with established HF is less clear due to the observed obesity survival paradox.1 For patients with obesity and heart failure with reduced ejection fraction (HFrEF), treatment of obesity using lifestyle,3,4 pharmacologic,5 and bariatric surgery6,7 strategies may improve symptoms, functional status, comorbidity burden and access to heart transplantation.8 Retrospective cohort studies also show that bariatric surgery can reduce medium-term mortality among patients with established HF (Table 1).
Table 1: Risk of Mortality After Bariatric Surgery for Obesity in Observational Studies Including a HF Patient Group
| Author, Journal, Year | Surgical Group | Comparator Group | Odds or Hazard Ratio for Mortality |
| Aleassa et al. Surg Obes Relat Dis 2019;15:1189-1196. | Inpatients with primary diagnosis of HF and a prior bariatric surgery, n=2810 | Matched 1:5 with similar inpatients with no history of bariatric surgery | OR 0.52 (95% CI 0.35-0.77) |
| Han et al. Surg Obes Relat Dis 2019;15:469-477. | Inpatients with primary diagnosis of HF and a prior bariatric surgery, n=3617 | Inpatients with primary diagnosis of HF and obesity without surgery | OR 0.47 (95% CI 0.37-0.74) |
| Höskuldsottir et al. J Am Heart Assoc 2021;10:e019323. (preexisting HF subgroup) | RYGB and preexisting HF n=142, Sweden, 34% female, 4.5-year follow-up | Matched non-surgical controls with obesity (matched to full cohort) | HR 0.23 (95% CI 0.12-0.43) |
| Doumouras et al. Circulation 2021;143:1468-1480. (preexisting HF subgroup) | RYGB/SG n=274 and preexisting HF, Canada, 61% female, 4-year follow-up | Propensity score matching for non-surgical controls (matched to a HF subgroup) | HR 0.43 (95% CI 0.24-0.78) |
Treating Advanced HF in Patients with Obesity
For patients with end-stage HFrEF, obesity has been associated with modestly reduced survival after heart transplantation (HT).9,10 Therefore the International Society of Heart and Lung Transplantation (ISHLT) guidelines consider BMI ≥35 kg/m2 a relative contraindication for HT. Consequently, the only advanced HF therapy option for many patients with severe obesity is left ventricular assist device (LVAD) implantation or palliative care, with some programs having no upper threshold of BMI pre-LVAD implantation. It is therefore not unusual for patients with severe obesity and advanced HF to receive an LVAD as destination therapy in the United States,11 with the intention of readdressing HT candidacy after sufficient weight loss during LVAD support.
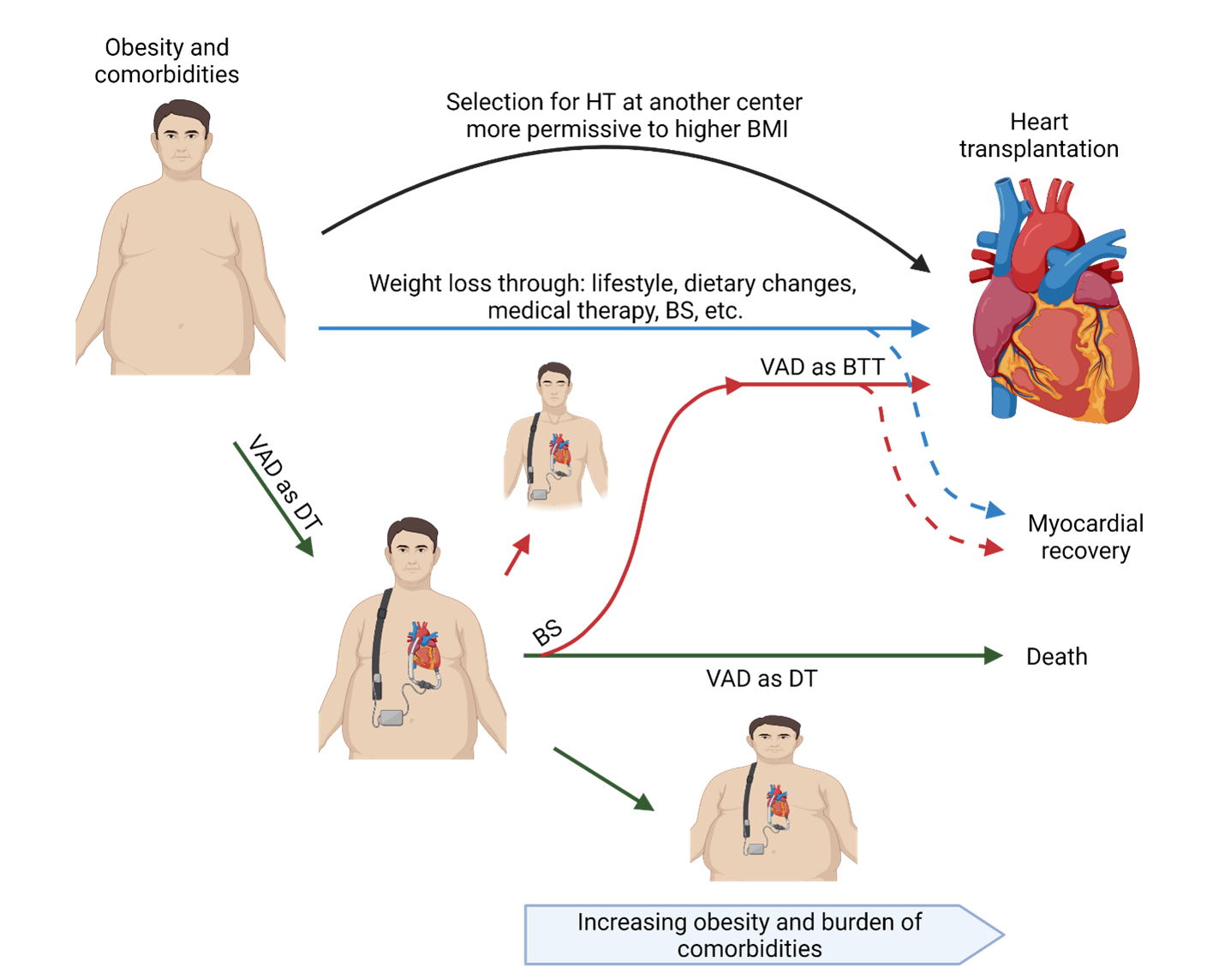
However, LVAD recipients tend to gain weight, which may thwart this 'bridge to transplant candidacy' strategy.12 The tendency towards weight gain has been attributed to a combination of increased appetite and reduced catabolic metabolism during LVAD support of the underlying HF syndrome, as well as limited exercise tolerance due to a combination of the HF, excess adiposity, and associated comorbidities. Therefore, LVAD implantation can incite a cycle of further weight gain during LVAD support and diminishing opportunities to achieve the HT goal (Figure 1).
Figure 1: Potential alternatives for patients with obesity to achieve heart transplantation
LVAD Outcomes for Patients with Obesity
In an analysis of 17,095 INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) registry participants who received LVADs 2006-2014, obesity was associated with lower likelihood of HT, especially among patients with BMI ≥35 kg/m2 (HR 0.571).13 Obesity was also associated with higher risks of infection, device malfunction/thrombosis, cardiac arrhythmias, and hospital readmissions, but a lower risk of bleeding complications. There was no association between obesity and mortality. Significant weight loss (≥10%) was achieved by only 18.6% of patients with initial BMI ≥35 kg/m2 and was associated with a higher chance of HT. Another cohort from the ISHLT Mechanically Assisted Circulatory Support (IMACS) registry included 9,408 continuous flow LVAD recipients implanted 2013-2015 and also showed an excess of device malfunction, device thrombosis, infection, and respiratory failure for recipients with BMI ≥40 kg/m2 and no mortality differential between BMI groups.14 A large meta-analysis (n=26,842) similarly associated BMI ≥30 kg/m2 with device-related infections, pump thrombosis and right heart failure; patients with obesity had lower mortality at 6 and 12 months of LVAD support, but no difference at 2 years.15 Device-specific data from the HeartWare ventricular assist device (HVAD) and HeartMate 3 (HM3) approval trials both also associated obesity with a higher rate of driveline infections.16,17
Weight Loss Strategies for Patients with LVADs
Despite reassuring survival outcomes, the excess in adverse events for LVAD recipients with baseline obesity paints a compelling case for formulating individualized weight management strategies. Specific data regarding dietary, exercise, pharmacological and bariatric surgery interventions in the advanced HF/LVAD population are sparse, but a comprehensive multi-specialty approach has been proven successful.18 This non-randomized study of 70 patients indicated that a multimodal intervention including dietary counselling plus psychosocial support may prevent weight gain after LVAD implantation, as well as improving exercise capacity and anxiety scores over 18 months.18 The feasibility and safety of exercise during continuous flow LVAD support was evaluated by the Rehab-VAD trial (n=26), demonstrating significant improvements in peak oxygen consumption, treadmill time, 6-minute walk distance and quality of life with a cardiac rehabilitation program consisting of 18 aerobic exercise visits, with only one adverse event of syncope, but changes in weight were not reported.
Several centers have reported their experiences with bariatric surgeries, either in staged or simultaneous fashions, for LVAD recipients (Table 2). The Ochsner Clinic team recently published a meta-analysis (n=29) of LVAD recipients with obesity (mean preoperative BMI 45.5 ± 6.6 kg/m2) who underwent bariatric surgery with significant weight loss.19 The composite outcome of BMI of <35 kg/m2, HT, listing for HT, or myocardial recovery was achieved by 78.6% of patients at 11 (3-17) months, without any deaths during HT-free 1-year follow up. Notably, all patients with preoperative BMI <45 kg/m2 achieved the composite outcome, versus 50% of patients with higher BMIs. Despite the short follow-up, 13 patients had undergone HT at 14.4 (± 7.0) months. In another meta-analysis (n=59) BMI decreased from 46.7 kg/m2 to 33.4 kg/m2 at most recent follow-up, with no differences between the simultaneous and staged strategies for postoperative complications or survival.20 The postoperative complication rate was 14.3%, which increased to 50% (4/8) in a single center series.
Table 2: Published Studies (with n ≥5) of Patients with Continuous Flow LVADs Undergoing Bariatric Surgery
| Author | BS type | Year of BS | n | Age, years | VAD type | Time from VAD implantation to BS, months | Preoperative mean BMI, kg/m2 | LOS, days | Adverse events | BMI < 35 kg/m2 | Listing for HT | HT | 30-day mortality | 1-year mortality | Length of follow up after BS, months | BMI at last follow up, kg/m2 |
| Hawkins RB, et al. | LSG | 2013-2017 | 11 | mean 43.3 (range 31–66) | - | 16 (range 5-32) | 45.2 (range 39-58) | median 9 | 1/11 | 7/11 | 7/11 | 4/11 | 0/11 | 1/11 | 12 (6–39) | mean 33.1 (range 26-39) |
| Punchai S, et al. | LSG | 2013-2017 | 7 | 39 (range 26-62) | - | 38 (range 15-48) | 43.6 (range 36.7-56.7) | 9 (range 6-23) | 5/7 | 4/7 | 3/7 | 0/7 | 0/7 | 24 (range 2-30) | 37.2 (range 24.3-46.3) | |
| Zenilman A, et al. | LSG | 2014-2018 | 6 | 48.3 (range 22.7-61.2) | HM2: 5 HVAD: 1 |
20 (range 9-21) | 41.4 (range 37.1-58) | 5.5 (range 3-7) | 1/6 | 4/6 | 4/6 | 3/6 | 0/6 | - | 19.5 (range 7.3-40.4) | 32.5 (range 23.1-51.4) |
| daSilva-deAbreu A, et al. | LSG | 2016-2020 | 8 | 43.8 (±13.9) | HM2: 3/8 HM3: 4/8 HVAD: 1/8 |
29.4 (±16) | 42.7 (±4.5) | 17 (±6.3) | 5/8* | 6/8 | 3/8 | 1/8 | 0/8 | 0/8 | 23.1 (±16.4) | 35.3 (±4.3) at 6 months |
| Challapalli J, et al. | LSG | 2000-2018 | 59 | 46 (CI 39-53) | 23.9 (CI 14.0-33.9) | 46.7 (CI 42.9-50.6) | 10.8 (CI 3.9-17.8) | 14.3% | - | 66% (CI 51-79) | 33% (CI 22-47) | - | 11.3%ⴕ | 12.7 (CI 6.3-19) | 33.4 (CI 30.2–36.6) | |
| daSilva-deAbreu A, et al. | LSG (82.8) RYGB (17.2%) | - | 29 | 41.9 (±12.2) | HM2: 8/10 HM3: 1/10 HVAD: 1/10 |
28.4 (±12.1) | 45.5 (±6.6) | 7 (IQR 5-10) | 39.3% | - | 78.3% | 46.4% | 0 | 0‡ | 24 (IQR 12-30) | - |
Optimizing LVAD Support for Patients with Obesity: Expert Perspective
Registry analyses suggest that obesity has minimal impact on medium-term survival during LVAD support but may confer higher rates of device infection and pump thrombosis, and lower likelihood of achieving eligibility for transplantation. Given the tendency for weight gain during LVAD support, it is advisable to engage patients with BMI ≥30-35 kg/m2 in a multi-modal and multi-disciplinary treatment plan for weight management and cardiovascular wellness. This individualized plan should ideally include a registered dietitian, psychosocial support, and cardiac rehabilitation components, and be instituted pre-implantation. The relative risks and benefits of the simultaneous versus staged approaches has not been systematically studied, but it should be noted that the patient education and lifestyle adjustments are considerable both for LVAD and bariatric surgery. Clinicians should also be aware of the potential for abnormal warfarin pharmacodynamics in LVAD recipients post-bariatric surgery, with a risk of over-anticoagulation and bleeding, or abnormal immunosuppressant absorption post-HT, with a risk of rejection. Malabsorption is generally greater with Roux-en-Y gastric bypass versus the more common sleeve gastrectomy. Prospective trials of simultaneous versus staged continuous flow LVAD support and bariatric surgery, as well as the alternate option of temporary mechanical support peri-bariatric surgery, are warranted to define best management strategies for patients with obesity and advanced HF.
References
- Mahajan R, Stokes M, Elliott A, et al. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta-analysis. Heart 2020;106:58-68.
- Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation 2017;135:1577-85.
- Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC. Reduced body weight and adiposity with a high-protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. J Cardiovasc Nurs 2009;24:207-15.
- Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36-46.
- Beck‐da‐Silva L, Higginson L, Fraser M, Williams K, Haddad H. Effect of orlistat in obese patients with heart failure: a pilot study. Congest Heart Fail 2005;11:118-23.
- Blumer V, Greene SJ, Ortiz M, et al. In-hospital outcomes after bariatric surgery in patients with heart failure. Am Heart J 2020;230:59-62.
- Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric surgery and emergency department visits and hospitalizations for heart failure exacerbation: population-based, self-controlled series. J Am Coll Cardiol 2016;67:895-903.
- Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail 2019;25:380-400.
- Russo MJ, Hong KN, Davies RR, et al. The effect of body mass index on survival following heart transplantation: do outcomes support consensus guidelines? Ann Surg 2010;251:144-52.
- Clerkin KJ, Naka Y, Mancini DM, Colombo PC, Topkara VK. The impact of obesity on patients bridged to transplantation with continuous-flow left ventricular assist devices. JACC Heart Fail 2016;4:761-68.
- Jaiswal A, Truby LK, Chichra A, et al. Impact of obesity on ventricular assist device outcomes. J Card Fail 2020;26:287-97.
- Emani S, Brewer RJ, John R, et al. Patients with low compared with high body mass index gain more weight after implantation of a continuous-flow left ventricular assist device. J Heart Lung Transplant 2013;32:31-35.
- Gadela NV, Umashanker D, Scatola A, Jaiswal A. Clinical outcomes, trends in weight, and weight loss strategies in patients with obesity after durable ventricular assist device implantation. Curr Heart Fail Rep 2021;18:52-63.
- Forest SJ, Xie R, Kirklin JK, et al. Impact of body mass index on adverse events after implantation of left ventricular assist devices: an IMACS registry analysis. J Heart Lung Transplant 2018;37:1207-17.
- Khan MS, Yuzefpolskaya M, Memon MM, et al. Outcomes associated with obesity in patients undergoing left ventricular assist device implantation: a systematic review and meta-analysis. ASAIO J 2020;66:401-08.
- Kiernan MS, Najjar SS, Vest AR, et al. Outcomes of severely obese patients supported by a centrifugal-flow left ventricular assist device. J Card Fail 2020;26:120-27.
- Patel CB, Blue L, Cagliostro B, et al. Left ventricular assist systems and infection-related outcomes: a comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant 2020;39:774-81.
- Kugler C, Malehsa D, Schrader E, et al. A multi-modal intervention in management of left ventricular assist device outpatients: dietary counselling, controlled exercise and psychosocial support. Eur J Cardiothorac Surg 2012;42:1026-32.
- daSilva-deAbreu A, Alhafez BA, Curbelo-Pena Y, et al. Bariatric surgery in patients with obesity and ventricular assist devices considered for heart transplantation: systematic review and individual participant data meta-analysis. J Card Fail 2021;27:338-48.
- Challapalli J, Maynes EJ, O'Malley TJ, et al. Sleeve gastrectomy in patients with continuous-flow left ventricular assist devices: a systematic review and metaanalysis. Obes Surg 2020;30:4437-45.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Prevention, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Acute Heart Failure, Heart Transplant, Mechanical Circulatory Support, Exercise
Keywords: Heart Failure, Body Mass Index, Quality of Life, Exercise Tolerance, Warfarin, Heart-Assist Devices, Obesity, Morbid, Gastric Bypass, Weight Loss, Cardiac Rehabilitation, Patient Readmission, Adiposity, Palliative Care, Retrospective Studies, Immunosuppressive Agents, Stroke Volume, Prospective Studies, Feasibility Studies, Follow-Up Studies, Psychosocial Support Systems, Heart Transplantation, Exercise, Gastrectomy, Thrombosis, Weight Gain, Postoperative Complications, Arrhythmias, Cardiac, Contraindications, Registries, Life Style, Lung Transplantation, Syncope, Respiratory Insufficiency, Comorbidity, Risk Factors, Oxygen Consumption, Risk Assessment, Anticoagulants, Counseling, Obesity
< Back to Listings