Risk-Guided Surveillance for Cardiomyopathy in Survivors of Childhood Cancer
Quick Takes
- In asymptomatic survivors of childhood cancer at risk for heart failure, a prediction model that uses anthracycline and chest-directed radiotherapy dose for the 10-year prediction of left ventricular systolic dysfunction (ejection fraction <40%) can be improved by including the left ventricular ejection fraction (LVEF) obtained from a surveillance echocardiogram at a median of 17 years from cancer diagnosis.
- The prediction model can be used to identify a large subgroup representing ±75% of the survivors with a predicted risk of ≤3% who are therefore unlikely to develop left ventricular systolic dysfunction within 10 years (negative predictive value 99%).
- This study1 adds evidence obtained from earlier simulation studies suggesting that cardiomyopathy surveillance can be reduced in low-risk survivors exposed to a cumulative anthracycline dose <100 mg/m2 and a chest-directed radiotherapy dose <15 Gray.
Introduction
Survivors of childhood cancer exposed to anthracyclines, mitoxantrone, and/or chest-directed radiotherapy are at increased risk for heart failure.2 Echocardiographic surveillance frequency recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) are currently based on cumulative exposures to anthracyclines and chest-directed radiotherapy.3 Whether intermediate echocardiographic results obtained during surveillance can refine future risk estimations and may be used to update surveillance frequency recommendations is a topic of interest.
Study Question
What is the added value of LVEF from an initial surveillance echocardiogram for predicting a clinically relevant LVEF <40% during follow-up in asymptomatic survivors of childhood cancer treated with cardiotoxic cancer therapies?
Methods
A retrospective study in two cohorts of long-term survivors of childhood cancer in the Netherlands was conducted. Inclusion criteria were previous exposure to cardiotoxic treatment (anthracyclines, mitoxantrone, and/or chest-directed radiotherapy), age ≥18 years, and survival of ≥5 years after cancer diagnosis at the first available surveillance echocardiogram. In addition, ≥2 follow-up echocardiograms had to be available not more than 5 years apart (in accordance with current IGHG recommendations). Exclusion criteria were heart failure symptoms or LVEF <40% before or at the initial surveillance echocardiogram.
Cox regression models were developed to predict risk for LVEF <40% within 10 years from the initial surveillance echocardiogram. Predictors were cumulative doses of anthracycline and chest-directed radiotherapy with or without addition of the initial surveillance LVEF. Findings were validated in an independent cohort.
Results
The derivation cohort included 299 survivors of childhood cancer with the initial surveillance echocardiogram performed at a median of 17 years from cancer diagnosis (interquartile range [IQR] 12-23). Median anthracycline dose was 280 mg/m2 (IQR 180-400), and median chest-directed radiotherapy dose was 25 Gray (IQR 18-33). Mean LVEF at the initial echocardiogram was 57% ± 7, and n = 41 (14%) had LVEF of 40-49%. Survivors in the validation cohort (n = 218) were treated with lower anthracycline doses (median 180 mg/m2) and had higher initial LVEF (mean 62%), and n = 12 (5.5%) had LVEF of 40-49%. Follow-up after the initial echocardiogram to last echocardiogram or LVEF <40% was longer in the derivation cohort (median 11 years; IQR 8-13) compared to the validation cohort (median 9; IQR 5-11).
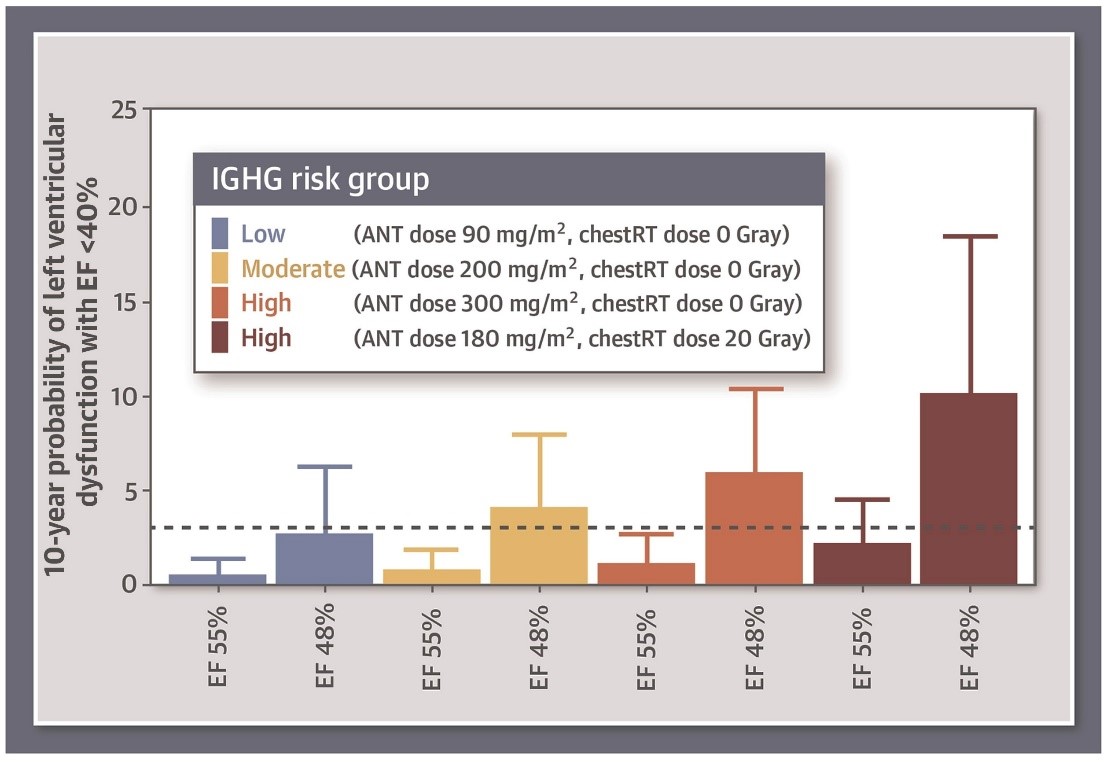
In the derivation cohort, the cumulative incidence of LVEF <40% during 10-years follow-up was 3.7% (11 events; 95% confidence interval [CI], 1.4-5.9). In multivariable Cox regression adjusted for anthracycline and chest-directed radiotherapy dose, the risk for developing LVEF <40% was higher in survivors with LVEF 40-49% compared to those with LVEF ≥50% (hazard ratio [HR] 7.81; 95% CI, 2.07-29.50). Higher anthracycline doses (HR per 100 mg/m2 increment of 1.70; 95% CI, 1.22-2.36) and higher chest-directed radiotherapy doses (HR per 10 Gray increment of 1.91; 95% CI, 1.34-2.72) were also associated with a higher risk for developing LVEF <40%. Addition of continuous LVEF to the Cox regression model resulted in an improved discrimination of LVEF <40% during follow-up (integrated area under the curve increase from 0.74 to 0.87; p < 0.001). There were a good calibration of the risk estimates and adequate validation of the model (integrated area under the curve increase from 0.72 to 0.86 in the validation cohort). Approximately 75% of the survivors in both cohorts had a 10-year risk of less than 3% for developing LVEF <40%, with a negative predictive value of 99% (95% CI, 98-100). Examples of predicted risk estimates for LVEF <40% within 10 years by IGHG risk group and mildly impaired versus normal LVEF at initial echocardiogram are shown in Figure 1.
Figure 1: Refinement of the IGHG Surveillance Guideline Risk Groups Using LVEF Measured With a Surveillance Echocardiogram
Conclusions
In survivors of childhood cancer, LVEF measured with a surveillance echocardiogram at a median of 17 years after cancer diagnosis improves the risk stratification for a therapeutically relevant decreased LVEF <40% in addition to anthracycline and chest-directed radiotherapy dose.
Perspective
This study demonstrates that LVEF results from a surveillance echocardiogram can be used to refine risk estimates for cardiomyopathy in long-term survivors of childhood cancer exposed to cardiotoxic cancer treatments (Figure 1). Results of this study add evidence to a recent simulation study showing that surveillance is not cost effective in survivors in the IGHG low-risk group (anthracycline dose <100 mg/m2 and chest-directed radiotherapy dose <15 Gray).4 In addition, the current study shows that survivors in the IGHG moderate risk group (anthracycline dose 100-249 mg/m2 or chest-directed radiotherapy dose 15-34 Gray) can be reclassified to a low-risk category based on LVEF. Although an increased risk of developing a LVEF <40% was demonstrated in survivors with an intermediate LVEF of 40-49% (HR 7.81; 95% CI, 2.07-29.50), the externally validated prediction model's main application is to reclassify ±75% of survivors into a low-risk category in whom surveillance frequency can potentially be safely reduced (i.e., once every 10 years). Replication in other cohorts is desirable, preferably from other countries. Further improvements in risk stratification are expected to come from additional predictors such as genetic variants, traditional cardiovascular risk factors, and other echocardiographic parameters, as well as different methods of modeling.
References
- Leerink JM, Van der Pal HJH, Kremer LCM, et al. Refining the 10-Year Prediction of Left Ventricular Systolic Dysfunction in Long-Term Survivors of Childhood Cancer. JACC CardioOncol 2021;3:62-72.
- Feijen EAM, Font‐Gonzalez A, Van der Pal HJH, et al. Risk and Temporal Changes of Heart Failure Among 5-Year Childhood Cancer Survivors: a DCOG-LATER Study. J Am Heart Assoc 2019;8:e009122.
- Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16:e123-36.
- Ehrhardt MJ, Ward ZJ, Liu Q, et al. Cost-Effectiveness of the International Late Effects of Childhood Cancer Guideline Harmonization Group Screening Guidelines to Prevent Heart Failure in Survivors of Childhood Cancer. J Clin Oncol 2020;38:3851-62.
Clinical Topics: Cardio-Oncology, Cardiovascular Care Team, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Acute Heart Failure, Echocardiography/Ultrasound
Keywords: Retrospective Studies, Mitoxantrone, Anthracyclines, Confidence Intervals, Predictive Value of Tests, Calibration, Follow-Up Studies, Cancer Survivors, Cardiovascular Diseases, Neoplasms, Risk Factors, Ventricular Dysfunction, Left, Echocardiography, Cardiomyopathies, Heart Failure, Heart Disease Risk Factors, Risk Assessment
< Back to Listings