Update in Prevention: 2021 Canadian Cardiovascular Society Dyslipidemia Guidelines
Quick Takes
- The new Canadian guidelines continue to recommend using the Framingham Risk Score rather than newer equations such as the pooled cohort equations to estimate ASCVD risk.
- When triglycerides are just >1.5 mmol/L (133mmol/L), the use of non-HDL-C and ApoB cutoffs are recommended to guide treatment decisions.
- Specific treatment thresholds for intensifying lipid lowering non-statin therapies are provided.
The Canadian Cardiovascular Society (CCS) has released new guidelines on management of dyslipidemia for the prevention of cardiovascular disease to replace the previous ones issued in 2016.1 While the overall approach to dyslipidemia with risk assessment followed by lifestyle and pharmacologic recommendations is similar to that of the 2018 American Heart Association (AHA)/American College of Cardiology(ACC)/Multisociety (MS) guideline, there are a number of notable differences.2
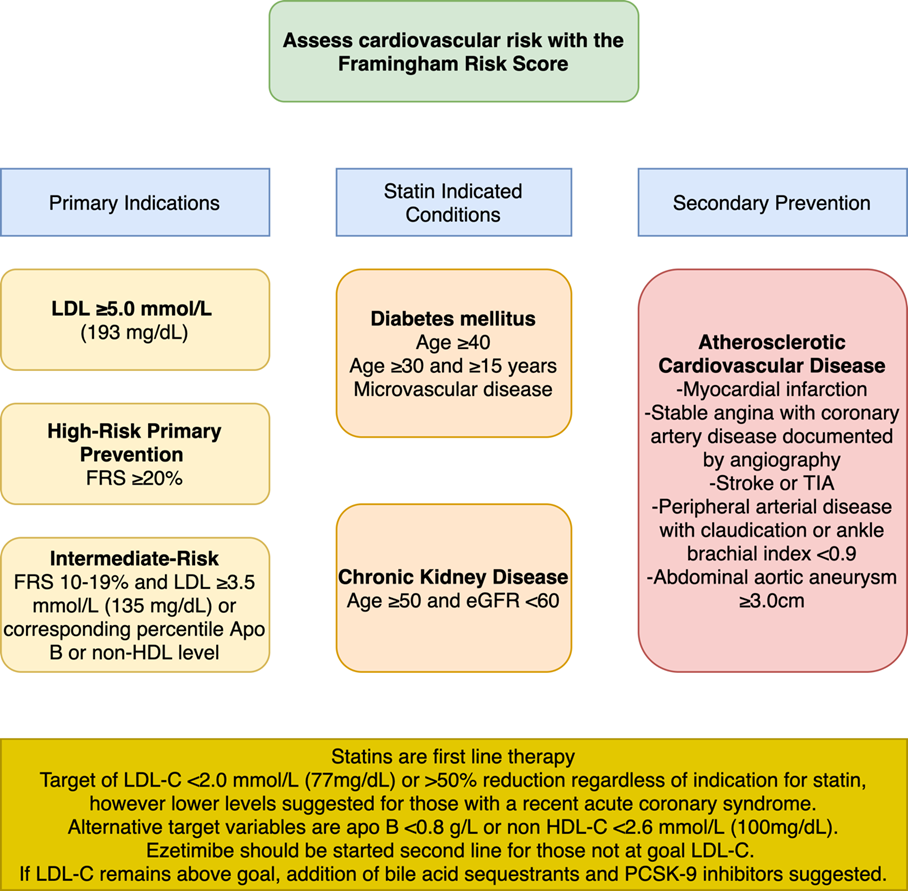
The CCS continues to recommend the Framingham Risk Score (FRS) to estimate risk in primary prevention, in contrast to the pooled cohort equations (PCE). The PCE were derived from Atherosclerosis Risk in Communities (ARIC), Coronary Artery Risk Development in Young Adults (CARDIA), Cardiovascular Health Study (CHS), as well as the Framingham original and offspring studies recommended by ACC/AHA/MS and also in contrast to Systematic Coronary Risk Estimation (SCORE) developed in European populations recommended by European Society of Cardiology (ESC).3 The recommendation for use of the FRS is notable, as this cohort was derived mainly from a mostly white population with higher exposure to environmental risk factors for atherosclerotic cardiovascular disease (ASCVD), such as tobacco use, than more modern cohorts. The FRS predicts fatal and nonfatal coronary heart disease (CHD), while the ASCVD risk estimator predicts coronary death, nonfatal myocardial infarction (MI), fatal or nonfatal stroke more precisely since many more adults are its database as compared to just the Framingham subjects alone.
Furthermore, it does not account for concomitant diabetes mellitus. The recommendation for risk estimation with the FRS presents an even higher bar for the use of high intensity statins or non-statin medications in primary prevention than the use of the PCE as the FRS does not consider stroke risk. It is thus possible that this may lead to significant underestimation of risk and under-treatment leading to a higher future burden of stroke and MI. This is especially important in Blacks who tend to have higher risk of stroke than whites, and therefore the lack of consideration of stroke in risk assessment may lead to missed opportunities for aggressive prevention with statin therapy and subsequent racial disparities.
These guidelines also place emphasis on the use of non-HDL-C and ApoB measurements, particularly in patients with triglycerides >1.5 mmol/L (133 mg/dL). These levels are incorporated throughout in recommended treatment thresholds. The rationale for this emphasis on non-HDL-C and ApoB is that LDL-C calculation by the traditional Friedewald equation is inherently inaccurate if hypertriglyceridemia exists. Also, the guideline acknowledges that most patients across Canada present for full lipid panels in the non-fasting state.
Non-HDL-C and ApoB are not significantly changed in the non-fasting state as compared to LDL-C. However, newer methods for LDL-C calculation are available to address this concern, most notably the Martin-Hopkins equation, which uses an adjustable factor for the triglyceride to VLDL-C ratio. It has been extensively validated in >1 million lipid profiles as more accurate than the Friedewald estimate; it consistently outperforms the Friedewald estimate when LDL-C is <100 mg/dL and when triglycerides are elevated.4-6 Putting less emphasis on LDL-C levels is somewhat surprising given that LDL-C is the principal evidence-based drug target from the clinical trials informing the guidelines. However, the CCS has modified values for ApoB and non-HDL-C from previous versions of their guidelines to represent the same percentile equivalents that were used traditionally for LDL-C.
The CCS recommends the initiation of statin therapy for primary prevention in those with an LDL-C of ≥5 mmol/L and those with a statin indicated condition such as diabetes mellitus or chronic kidney disease (except those receiving dialysis) regardless of estimated FRS risk. The initiation of a statin is also recommended for individuals with an estimated 10-year FRS risk of ≥20% (high risk), and those 10-19.9% (intermediate FRS risk) who have LDL-C greater than 3.5 mmol/L (135 mg/dL), non-HDL-C >4.2 mmol/L (162 mg/dL), or ApoB >1.05 g/dL. Men ≥50 and women ≥60 with other risk factors such as hypertension, impaired glucose tolerance, smoking, central adiposity, or other risk modifiers such as hsCRP ≥ 2.0mg/L, non-zero coronary artery calcification, Lp(a) ≥50 mg/dL (100 nmol/L) or family history of premature CHD are also classified as intermediate risk for whom a statin is recommended.
Another unique aspect of the newly released CCS guidelines is a focus on identifying and treating women with high-risk conditions of pregnancy to decrease their long term ASCVD risk. Complications of pregnancy such as preeclampsia, intrauterine growth restriction, hemolysis with elevated liver enzymes and low platelets, and other severe complications are associated with higher lifetime risk of developing ASCVD risk factors such as hypertension, diabetes mellitus, dyslipidemia, metabolic syndrome, and subclinical atherosclerosis. The AHA/ACC/MS guidelines also emphasized conditions specific to women such as pre-eclampsia and premature menopause as predictors of future adverse ASCVD outcomes.
The CCS guidelines recommend screening these women with a lipid panel in the postpartum period and early counseling to engage in lifestyle modification. Notably, the authors recommend favoring cardiovascular age or a lifetime risk estimate over 10-year risk calculators in this population, although the authors note that only low-quality evidence exists to do so. On the question of treatment, the authors suggest the use of hydrophilic statins such as rosuvastatin or pravastatin given that most cases of fetal harm occurred with the use of lipophilic statins, which theoretically can more readily cross the placental barrier.7
The CCS recommends lipoprotein(a) (Lp[a]) measurement for everyone once. However, Lp(a) assay standardization is still a work in progress.8 We do not have data that treating patients with an ASCVD risk estimate <5% and an elevated Lp(a) with a statin is cost-effective. Also, the cutoffs for a high Lp(a) are likely different between ethnic groups.
These new recommendations of dyslipidemia management reflect a rapidly evolving body of research in a quickly changing field. With new therapies such as inclisiran, bempedoic acid, pelacarsen and pemafibrate all currently undergoing evaluation in randomized controlled trials, we can expect many more advances and updates from the CCS and other organizations in the next few years.
Figure 1
References
- Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37:1129-50.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285-e350.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88.
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013;310:2061-68.
- Martin SS, Giugliano RP, Murphy SA, et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol 2018;3:749-53.
- Sathiyakumar V, Park J, Golozar A, et al. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation 2018;137:10-19.
- Pollack PS, Shields KE, Burnett DM, Osborne MJ, Cunningham ML, Stepanavage ME. Pregnancy outcomes after maternal exposure to simvastatin and lovastatin. Birth Defects Res A Clin Mol Teratol 2005;73:888-96.
- Wilson DP, Jacobson TA, Jones PH, et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374-92.
Clinical Topics: Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Prevention, Advanced Lipid Testing, Hypertriglyceridemia, Lipid Metabolism, Nonstatins, Novel Agents, Statins, Hypertension, Smoking
Keywords: Dyslipidemias, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Rosuvastatin Calcium, Pravastatin, Pre-Eclampsia, Cholesterol, LDL, C-Reactive Protein, Cardiovascular Diseases, American Heart Association, Lipoprotein(a), Metabolic Syndrome, Glucose Intolerance, Coronary Vessels, Apolipoproteins B, Triglycerides, Ethnic Groups, Cost-Benefit Analysis, Menopause, Premature, Hemolysis, Canada, Adiposity, African Americans, Blood Platelets, Renal Dialysis, Atherosclerosis, Primary Prevention, Diabetes Mellitus, Life Style, Coronary Disease, Risk Assessment, Risk Factors, Hypertriglyceridemia, Hypertension, Myocardial Infarction, Renal Insufficiency, Chronic, Pharmaceutical Preparations, Stroke, Tobacco Use, Smoking, Counseling, Postpartum Period, Reference Standards, Pregnancy
< Back to Listings