COVID-19, Myocarditis, and Cardiac MRI in Athletes: Distinguishing Signal from Noise
Quick Takes
- Studies of cardiac injury in athletes infected with SARS-Co-V2 have shown variable prevalence estimates of cardiac involvement ranging from 0-15% with no reported instances of sudden cardiac death.
- Cardiac MRI (CMR) is most useful in athletes with high pretest probability for SARS-CoV-2 cardiac involvement based on the presence of cardiopulmonary symptoms and/or abnormalities on cardiac testing. A universal screening approach with CMR in COVID-19-positive athletes places unnecessary stress on healthcare resources with potential for false positives and harm without known benefit.
- Management decisions regarding return to play and risk-acceptance should be made in conjunction with athletes and on a case-by-case basis with a patient-centered approach.
Post-viral myocarditis is a well-documented cause of sudden cardiac death in athletes.1,2 Myocarditis is often suspected but rarely confirmed by endomyocardial biopsy (EMB), which has created a need for noninvasive diagnostic criteria to guide recommendations for athletic participation and return to play (RTP) protocols.
Acute myocarditis is diagnosed by the presence of both a clinical syndrome, that includes acute heart failure, angina-type chest pain, or myopericarditis of <3 months duration, and an otherwise unexplained elevation in serum troponin, electrocardiographic features of cardiac ischemia, high-degree AV block or arrhythmias, and/or wall motion abnormalities with or without pericardial effusion on echocardiography or cardiac MRI (CMR).3 The 2018 Lake Louise Criteria expanded the diagnostic role of CMR, noting alterations in tissue signal on T2- or T1-weighted images and presence of late gadolinium enhancement (LGE) could indicate myocarditis in symptomatic individuals.4
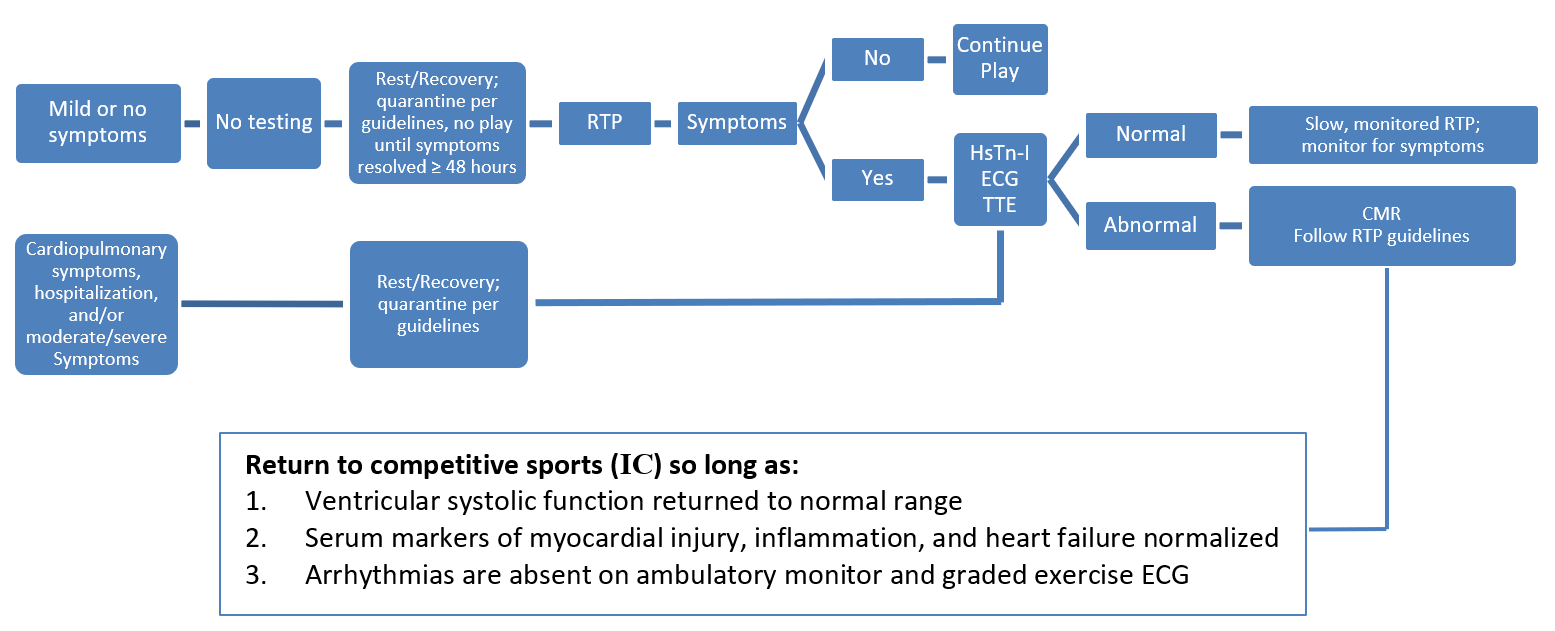
During the 2019 novel coronavirus (COVID-19) pandemic, data showing myocardial involvement in infected individuals, as well as popular news stories of high-profile athletes being sidelined by presumptive COVID-19-related myocarditis, raised concerns about the safety of allowing athletes to RTP after COVID-19 infection.5,6 Experts currently recommend a symptom-based return to exercise and imaging for those with moderate to severe symptoms, especially cardiopulmonary symptoms (Figure 1).7
Figure 1: RTP Guidelines in COVID-19-Positive Athletes. Courtesy of Gagel AC, Sharma G, Blumenthal RS, Martinez MW. Adapted from Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after Coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol 2020;5:1085-86.
CMR in COVID-19 Positive Athletes - The Data Thus Far
Multiple studies of cardiac outcomes in athletes previously infected with SARS-Co-V2 have been conducted since September 2020 (Table 1) and have shown variable prevalence estimates of cardiac involvement ranging from 0-15%. Most studies included a CMR-based screening approach utilizing the Lake Louise Criteria in aiding diagnosis. Two studies noted positive CMR findings by Lake Louise Criteria in asymptomatic athletes with previous negative cardiac testing. A single study revealed a high prevalence of pericardial involvement, which is discordant from findings reported in other studies. Reassuringly, no deaths were attributed to COVID-19-related myocarditis in these studies.
Table 1: Studies in COVID-19-Positive Athletes, Summary of Key Findings. Courtesy of Gagel AC, Sharma G, Blumenthal RS, Martinez MW.
| Study | Participants | Athletes | Findings |
| Rajpal et al.8 | 26 | Collegiate | All had normal TnI level and no ECG or TTE findings; 15% had CMR findings suggestive of myocarditis (T2 signal an LGE); 2 athletes with +CMR were asymptomatic. |
| Vago et al.9 | 12 | Professional | All had normal TnI levels and no cardiac symptoms; CMR showed no myocardial or pericardial edema or LGE. |
| Clark et al.10 | 59 | Collegiate | All had normal ECG, TnI, and TTE; 3% met criteria for myocarditis. |
| Malek et al.11 | 26 | Professional | 15% had detectable Tn-I. CMR abnormalities found in 5 (19%) athletes, 4 with isolated myocardial edema and 1 with LGE with pleural and pericardial effusion. CMR did not reveal any case of acute myocarditis. |
| Starekova et al.12 | 145 | Collegiate | 2 (1.4%) had cardiac MRI findings consistent with myocarditis. 81.4% were symptomatic with 4.8% experiencing chest pain. |
| Brito et al.13 | 54 | Collegiate | 1 had elevated Tn-I levels. 46 of 54 underwent CMR. 27 of 46 (56.3%) had abnormal CMR findings. 19 (39.5%) had pericardial late enhancements with associated pericardial effusion. Native T2 findings were normal in all; no specific imaging features of myocardial inflammation identified. |
| Martinez et al.14 | 789 | Professional | Abnormal screening identified in 30 (3.8%; tn-I, 6 [0.8%]; ECG, 10 [1.3%]; TTE, 20 [2.5%]). 27 had CMR; 5 (0.6%) had imaging evidence of inflammatory heart disease. |
| Moulson et al.15 | 3,018 | Collegiate | 13% had cardiopulmonary symptoms. 21/2999 had abnormal (0.7%), 24/2719 had elevated Tn-I (0.9%), and 24/2556 had abnormal TTE (0.9%). 198 athletes had CMR. 3/198 (1.5%) had definite or probable cardiac involvement. |
| Hendrickson et al.16 | 137 | Collegiate | Tn-I levels abnormal in 4 (2.9%). There were no ECG or TTE abnormalities; 5 underwent CMR. None had abnormal findings detected by CMR. |
| Daniels et al.17 | 1,597 | Collegiate | CMR done in all COVID-19-positive athletes regardless of cardiac symptoms or other cardiac testing results, increasing the prevalence of detecting myocarditis to 2.3%. |
| CMR: Cardiac MRI, TnI: Troponin-I, TTE: Transthoracic Echocardiogram, LGE: Late Gadolinium Enhancement, ECG: Electrocardiogram | |||
Challenges in Differentiating the Athlete's Heart from Possible COVID-19 Cardiac Injury
"Abnormal" findings concerning for myocarditis can be normal variants in athletes, and several clinical challenges may arise in differentiating normal exercise-induced cardiac changes from pathologic insults as a consequence of COVID-19 infection. In healthy individuals, exercise can lead to transient elevations in troponin and short-term myocardial inflammation and tissue edema by CMR. Echocardiographic studies in endurance athletes have shown left ventricle (LV) dilation, reduced left ventricular ejection fraction (LVEF), and right ventricular remodeling, and several studies have suggested increased prevalence of fibrosis in endurance athletes above 30 years of age (with no increased association of arrhythmia).18-24 Furthermore, in a CMR study from Clark et al., when COVID-19-positive athletes were compared to retrospective controls, focal LGE at the right ventricle insertion was present in 22% of study subjects compared to an identical LGE pattern in 24% of athletic controls.10 Thus, with current data, it remains difficult to determine if abnormal diagnostic, echocardiographic, and CMR findings in COVID-19 positive athletes are truly abnormal and concerning versus normal variants of the athletic heart.
Appropriate Screening in COVID-19-Positive Athletes
Potential cardiac injury in COVID-19 patients is concerning and warrants diagnostic investigation. Symptomatic individuals diagnosed with myocarditis of any etiology have higher rates of all-cause mortality independent of clinical symptoms, though it is unclear whether these rates extend to athletes with asymptomatic or mildly symptomatic disease.25
Daniels et al. reported CMR in all athletes testing positive for COVID-19 by polymerase chain reaction, regardless of symptoms, yielded a 7.4-fold increase in diagnosing myocarditis compared to a symptom-driven strategy and a 2.8-fold increase compared to standard triad testing (ECG, echocardiogram, troponin).17 However, Moulson et al. noted the diagnostic yield of CMR was 4.2 times higher for a clinically indicated CMR versus a screening CMR.15 Of note, the Lake Louise Criteria were developed for patients with a clinical presentation consistent with myocarditis, not asymptomatic individuals. The specificity of current CMR myocarditis criteria in an asymptomatic and otherwise healthy population is unknown and cannot be confidently applied to athletes with low clinical test probability for myocarditis.26
Studies to date support current recommendations that asymptomatic or mildly symptomatic athletes fully recovered from SARS-CoV-2 infection without cardio-pulmonary symptoms may return to sport without cardiac testing. Cardiac evaluation including ECG, troponin, and transthoracic echocardiography (TTE) should be considered in athletes with moderate-to-severe and/or cardiopulmonary symptoms during initial illness or upon return to exercise.7 CMR is most useful in athletes with high pretest probability for SARS-CoV-2 cardiac involvement based on the presence of cardiopulmonary symptoms and/or abnormalities on cardiac testing. Despite the use of CMR for the diagnosis of myocarditis, current RTP guidelines do not advocate using CMR when making RTP decisions (see Figure 1).7 Moreover, it is unresolved whether resolution of myocarditis related LGE should be required to permit return to competitive sports.4
Conclusion
As use of CMR increases in the evaluation of athletes convalesced from COVID-19, caution is required when determining the screening utility of CMR in the absence of diagnostic specificity. It also remains unclear whether isolated cardiac abnormalities detected by CMR harbor a risk like clinically diagnosed myocarditis.
CMR is an expensive resource requiring a level of expertise for interpretation not widely available. Incorporating a universal CMR screening approach in COVID-19-positive athletes would place stress on healthcare resources with likely little clinical benefit. Lessons learned from the introduction of ECG screening for athletes show that, in the absence of standardized measurements and normative data, use of CMR may result in inordinate false-positive rates, leading to unnecessary downstream testing and unwarranted medical disqualifications.27 Future recommendations are expected to provide improved guidance regarding whom to evaluate with cardiac testing and how to best utilize CMR to confirm cardiac injury in those COVID-19-positive athletes.
Unfortunately, screening can lead to both false-positive and false-negative findings, and there are challenges with the interpretation of biomarkers, ECGs, and imaging tests in athletes in whom exercise-induced cardiac remodeling can be misinterpreted as pathology. As per the American Society of Echocardiography, optimal use of multimodality imaging in athletes requires both an understanding of expected physiologic changes in athletic hearts and the strengths and weaknesses of available imaging techniques to integrate and interpret multimodality diagnostic imaging.20 With uncertainties present, management decisions regarding RTP and risk-acceptance should be made in conjunction with athletes using a patient-centered approach.
References
- Peterson DF, Kucera K, Thomas LC, et al. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: a 4-year prospective study. Br J Sports Med 2020;Nov 12:[Epub ahead of print].
- Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States National Registry. Am J Med 2016;129:1170-77.
- Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2362-2371.
- Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158-76.
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265-73.
- Lavigne P, Schlabach M. Heart condition linked with COVID-19 fuels Power 5 concern about season's viability (ESPN website). 2020. Available at: https://www.espn.com/college-football/story/_/id/29633697/heart-condition-linked-covid-19-fuels-power-5-concern-season-viability . Accessed 10/05/2020.
- Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after Coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol 2020;5:1085-86.
- Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021;6:116-18.
- Vago H, Szabo L, Dohy Z, Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. JACC Cardiovasc Imaging 2021;14:1279-81.
- Clark DE, Parikh A, Dendy JM, et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation 2021;143:609-12.
- Małek ŁA, Marczak M, Miłosz-Wieczorek B, et al. Cardiac involvement in consecutive elite athletes recovered from COVID-19: a magnetic resonance study. J Magn Reson Imaging 2021;Jan 20:[Epub ahead of print].
- Starekova J , Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol 2021;6:945-50.
- Brito D, Meester S, Yanamala N, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging 2021;14:541-55.
- Martinez MW, Tucker AM, Bloom OJ, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol 2021;6:745-52.
- Moulson N, Petek BJ, Drezner JA, et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 2021;144:256-66.
- Hendrickson BS, Stephens RE, Chang JV, et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation 2021;143:1926-28.
- Daniels CJ, Rajpal S, Greenshields JT, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 Cardiac Registry JAMA Cardiol 2021;6:1078-87.
- Shave R, Baggish A, George K, et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol 2010;56:169-76.
- La Gerche A, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 2012;33:998-1006.
- Baggish AL, Battle RW, Beaver TA, et al. Recommendations on the use of multimodality cardiovascular imaging in young adult competitive athletes: a report from the American Society of Echocardiography in collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2020;33:523-49.
- Zhang CD, Xu SL, Wang XY, Tao LY, Zhao W, Gao W. Prevalence of myocardial fibrosis in intensive endurance training athletes: a systematic review and meta-analysis. Front Cardiovasc Med 2020;7:585692.
- Franzen E, Mangold S, Erz G, et al. Comparison of morphological and functional adaptations of the heart in highly trained triathletes and long-distance runners using cardiac magnetic resonance imaging. Heart Vessels 2013;28:626-31.
- Abdullah SM, Barkley KW, Bhella PS, et al. Lifelong physical activity regardless of dose Is not associated with myocardial fibrosis. Circ Cardiovasc Imaging 2016;9:e005511.
- Sanchis-Gomar F, Joyner MJ, Löllgen H, Lucia A. Confounders in the evaluation of cardiac fibrosis by late gadolinium enhancement. Sports Med 2016;46:1193-94.
- Gräni C, Eichhorn C, Bière L, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017;70:1964-76.
- Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158-76.
- Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol 2017;69:1057-75.
Clinical Topics: Arrhythmias and Clinical EP, COVID-19 Hub, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Pericardial Disease, Sports and Exercise Cardiology, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Echocardiography/Ultrasound, Magnetic Resonance Imaging, Sports and Exercise and Imaging
Keywords: COVID-19, SARS-CoV-2, Stroke Volume, Gadolinium, Heart Ventricles, Ventricular Remodeling, Contrast Media, Return to Sport, Myocarditis, Troponin, Retrospective Studies, Pericardial Effusion, Atrioventricular Block, Coronavirus, Dilatation, Ventricular Function, Left, Magnetic Resonance Imaging, Electrocardiography, Echocardiography, Death, Sudden, Cardiac, Death, Sudden, Cardiac, Athletes, Sports, Fibrosis, Heart Failure, Biopsy, Edema, Biomarkers, Reference Standards, Polymerase Chain Reaction, Patient-Centered Care, Inflammation, Probability, Chest Pain, Delivery of Health Care, Ischemia
< Back to Listings