Implantable Pulmonary Artery Catheter Monitoring System Performance Across Diverse Heart Failure Phenotypes: Does it Work For Your Patient?
Quick Takes
- There is no difference in outcomes among patients managed via implantable pulmonary artery catheter or standard clinical practice irrespective of NYHA class profile and ejection fraction range.
- Conflicting results may be a product of lower event rates seen during the COVID-19 pandemic time period of the study.
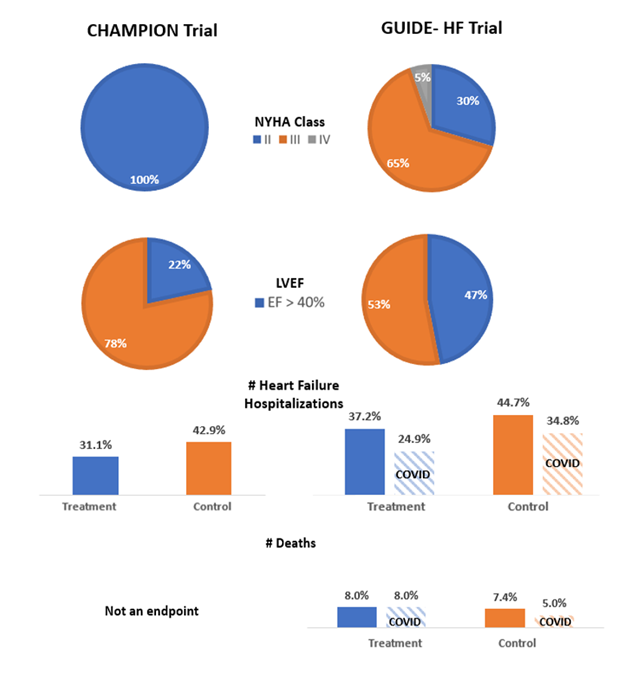
The GUIDE-HF trial: a randomized trial of hemodynamic-guided management of heart failure was published on August 27, 2021.1 It set out to assess the efficacy of hemodynamic-guided heart failure (HF) management based on data from implantable pulmonary artery pressure monitors (CardioMEMS™ HF system) across patients with all ejection fractions and New York Heart Association (NYHA) class II to IV HF symptoms. This contrasts with the landmark trial by Abraham et al. in 2011 that enrolled patients with NYHA class III symptoms and led to the device's approval for clinical use by the Food and Drug Administration in 2014.2 The GUIDE-HF trial is a single-blinded study which enrolled 1,000 patients across 118 centers from the United States and Canada and randomized them in a 1:1 fashion to either a hemodynamic-guided management strategy via pressure monitor data or usual care. Patients with stage D HF, those eligible to receive a heart transplant or left ventricular assist device in the next 12 months, and patients who received inotropes in the last 6 months were excluded. The study encompassed approximately a mean patient age of 71 with 38% female participants as well as 29%, 65%, and 5% of patients with NYHA class II, III and IV symptoms respectively. After a 12 month follow up period, the primary endpoint of all-cause mortality, HF hospitalization, or urgent HF visits was reached by 253/497 (0.56 per patient-year) in the hemodynamic-guided group and 289/502 (0.64 per patient-year) in the usual care group (Hazard Ratio [HR] 0.88, 95% confidence interval [CI] 0.74-1.05, p=0.16). Authors attributed some of the null effect to the COVID-19 pandemic acting as a time-dependent variable as 74% of the events occurred in the pre-COIVD era (up to March 13, 2020). The primary composite endpoint was reduced in favor of the hemodynamic-guided group (HR 0.81, 95% CI 0.66-1.00, p=0.05). This difference in primary events decreased during the COVID-19 pandemic. However, there may also be a confounding effect of different ejection fraction (EF) range given the broad inclusion criteria.
On November 6, 2021, additional study results with regards to EF stratification were presented at the Transcatheter Cardiovascular Therapeutics (TCT) Conference in Orlando, FL. The original study cohort had an average EF of 39% with 154/497 (31%) and 167/503 (33%) patients in the treatment and control groups respectively having an EF >50%. The primary endpoint for patients with an EF of <50% was HR 0.85, 95% CI 0.66-1.09 (p=0.20) and a HR 0.77, 95% CI 0.59-1.00 (p=0.051) for HF hospitalizations. Conversely, for those patients with an EF ≥50% the HR for the primary endpoint was 0.70, 95% CI 0.47-1.03 (p = 0.07) with a HR for HF hospitalization 0.72, 95% CI 0.48-1.07 (p = 0.11). Although results were similar for patients with reduced and preserved EF, there was a signal towards improved composite endpoints in the preserved EF group outside of HF hospitalizations. Implantable pulmonary artery pressure monitors remain one of the few HF therapies shown to be of benefit for patients with preserved EF. However, previous trials have mainly shown an improvement in HF hospitalizations with no prior survival analysis performed.3 This trial hints at a potential survival benefit in this population, although it did not reach statistical significance. Similar future trials will need to be performed given the possible effect of the COVID-19 pandemic on event rates.
Figure 1
References
- Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991-1001.
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658-66.
- Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935-44.
Clinical Topics: Cardiac Surgery, COVID-19 Hub, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Cardiac Surgery and Heart Failure, Acute Heart Failure, Heart Transplant, Mechanical Circulatory Support
Keywords: Stroke Volume, Follow-Up Studies, COVID-19, SARS-CoV-2, Confidence Intervals, Control Groups, Heart-Assist Devices, Pandemics, Pulmonary Artery, United States Food and Drug Administration, Heart Failure, Hospitalization, Heart Transplantation, Survival Analysis, TCT21, Transcatheter Cardiovascular Therapeutics
< Back to Listings