Pericardial Fat and Cardiomyopathy
Recent analyses from the MESA (Multi-Ethnic Study of Atherosclerosis), a large, community-based cohort of women and men, have turned the spotlight on the pathological influence of pericardial fat depot on the occurrence of newly diagnosed heart failure (HF).1,2 In this investigation, 6,785 study participants from four different races/ethnicities free of cardiovascular disease at baseline were followed for a median of 15.7 years during which time 385 individuals were newly diagnosed with HF. Multidetector computed tomography (CT) was used to estimate pericardial fat volume (PFV). The key findings of this study were as follows:
- After adjustment for established risk factors, pericardial fat volume (PFV) was associated with a greater risk of HF in both women and men.
- A greater amount of PFV was associated with a linear increase in the risk of HF without evidence of a threshold.
- The amount of PFV was lower in women than in men (69 cm3 vs. 92 cm3; p <0.001). The optimal cutoff of high versus normal PFV for HF prediction was therefore lower in women (≥70 cm3) than in men (≥120 cm3).
- The relative risk of newly diagnosed HF associated with greater PFV was higher in women than in men. Every one standard deviation (42 cm3) of excess PFV increased the risk of HF by 44% in women and 13% in men. High PFV approximately doubled the risk of HF in women and conferred about a 50% higher risk in men.
- Major risk factors of HF including hypertension, diabetes mellitus, dyslipidemia, and interim myocardial infarction explained about one-third of the association between elevated PFV and newly diagnosed HF in women and almost one-half in men.
- The association between PFV and newly diagnosed HF remained robust after accounting for anthropometric indicators of obesity such as body-mass index, waist circumference, hip circumference, and waist-to-hip ratio.
- CT-based estimates of abdominal subcutaneous fat, abdominal visceral fat, or hepatic fat (non-alcoholic fatty liver disease) did not substantially influence or mediate the association between PFV and HF risk in multivariable statistical models.
- The effect of PFV on the occurrence of HF was not attenuated by biomarkers of inflammation (C-reactive protein and interleukin-6) and hemodynamic stress (N-terminal pro-B-type natriuretic peptide).
- The strength of the association between PFV and incident HF was similar in White, Black, Hispanic, and Chinese Americans. Thus, race and/or ethnicity did not alter the association between pericardial fat and the development of HF.
- High compared with normal PFV was associated with a higher cumulative incidence of HF with preserved, mid-range, and unknown ejection fraction (heart failure with preserved ejection fraction [HFpEF], heart failure with mid-range ejection fraction [HFmrEF], and heart failure with unknown ejection fraction [HFuEF]) but not reduced ejection fraction (HFrEF). Of note, every one standard deviation (42 cm3) increase in PFV was associated with a 42% higher risk of HFpEF. High compared with normal PFV conferred a 2.3-fold greater risk of HFpEF. After adjustment for confounders (not intermediary variables), high PFV was associated with a 40% higher risk of HFrEF that approached near statistical significance. On further adjustment for variables in the causal pathway, the residual 20% elevated risk was not statistically significant.
In a subsample of the Jackson Heart Study (JHS) too, where CT-based estimation of PFV was comparable to that of the MESA cohort, a linear association between PFV and hospitalization for incident worsening HF was evident.3 During a median follow-up of 10.6 years, 77 of 1,386 Black participants were hospitalized for worsening HF. In fully adjusted multivariable models, every 10 cm3 increase in PFV was associated with an 8% increase in the risk of hospitalization for incident worsening HF. Further, in a smaller subset, a statistically significant association was noted between PFV and hospitalization for HFpEF but not HFrEF.
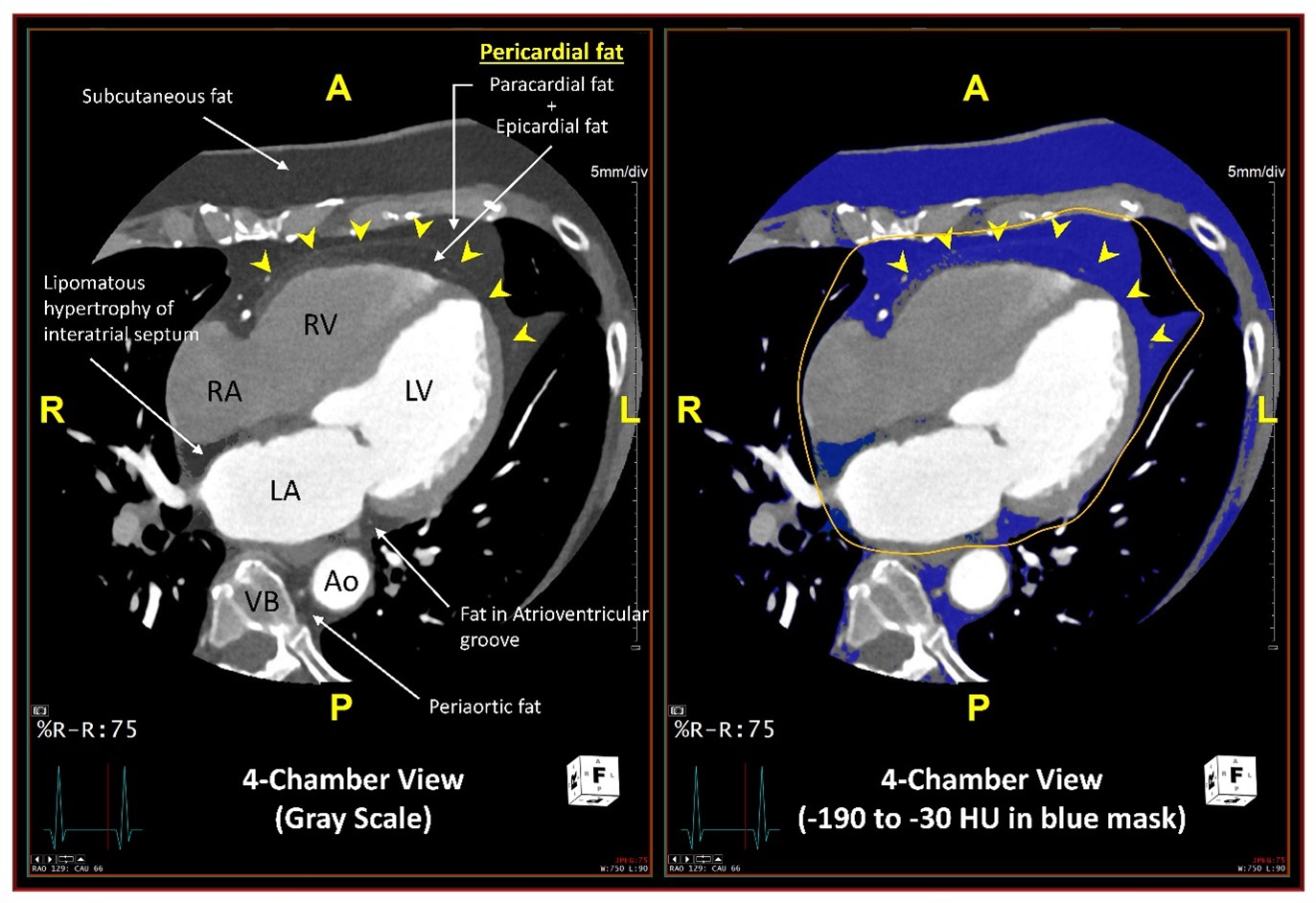
Of note, in both MESA and JHS, CT-based estimate of pericardial fat included a composite of epicardial fat, confined between the myocardium and the visceral pericardium, and paracardial (extrapericardial or mediastinal) fat, located external to the parietal pericardium (Figure 1). These two fat depots were not separately quantified due to difficulty in delineating the pericardium between them, particularly in lean individuals, and because the amount of these two fat depots were highly correlated in a subsample. However, epicardial and paracardial fat depots have inherent differences in their embryological origin, anatomic location, blood supply, and biochemical and biomolecular composition (Table 1).
Figure 1
A, P, R, and L indicate anterior, posterior, left and right orientations respectively. RA = right atrium; RV = right ventricle; LA = left atrium; LV = left ventricle; Ao = descending thoracic aorta; VB = vertebral body.
Table 1: Profiles of Epicardial and Paracardial Fat Depots
| Epicardial fat | Paracardial fat | |
| Embryology | ||
| Origin4 | Splanchnopleuric mesoderm | Primitive thoracic mesenchyme |
| Anatomy | ||
| Location in relation to the pericardium | Internal to visceral pericardium | External to parietal pericardium |
| Location in relation to the coronary arteries | Surrounds coronary arteries5 | Unrelated |
| Location in relation to the myocardium | Directly over the myocardium (no intervening fascia separating it from the myocardium)6 | Separated from the myocardium by visceral and parietal pericardium |
| Coverage around normal heart | 56% to 100% of the mid-cardiac circumference7 | Not well-described |
| Proportion of normal heart mass | 4% to 52% of heart mass7 ~20% of ventricular mass8 |
Not well-known |
| Proportion of intrathoracic fat9 | ~30% | ~70% |
| Blood supply in relation to the myocardium4 | Same (branches of coronary arteries) |
Unrelated (pericardiocophrenic branch of the internal mammary artery) |
| Microcirculation with coronary wall (vasa vasora) | Well-known4,8 | Not known |
| Adipocyte size | Smaller1 | Relatively larger10 |
| Brown adipose tissue features | 5-fold higher expression of Uncoupling Proten-1 (UCP-1) than in substernal fat12 | 24-fold higher expression of UCP-1 than in subcutaneous fat13; Focal increase in fluorodeoxyglucose (FDG) uptake14 |
| Physiology (Biochemical and Biomolecular) | ||
| Fatty acid synthesis (uptake) (lipogenesis)4 | Higher | Relatively lower |
| Fatty acid release (lipolysis)4 | Higher (two times that of paracardial fat) | Relatively lower |
| Energy consumption | High11 | Not well-known |
| Local source of energy for the myocardium | Plausible4,15 | Not known |
| Fatty acid composition | Lower C18:1 to C18:0 ratio10 High in saturated fat16 |
Higher C18:1 to C18:0 ratio10 |
| Protein content4 | Similar (two times that of perirenal and popliteal fat) | Similar |
| Glucose utilization | Lower (half that of intra-abdominal fat)4 | Not well-known |
| Paracrine and vasocrine function | Well-known source of adipokines;5,6,17 marginally lower adiponectin gene expression than in subcutaneous fat;11 expresses more inflammatory cytokines than subcutaneous fat18 | Not well-known |
Epicardial fat has been hypothesized to cushion coronary arteries against torsion,19 generate heat in response to hypothermia,12 serve as an energy repository for the myocardium,15 sequester excess fatty acids from the coronary circulation,15,20 enable positive coronary remodeling,21 produce anti-inflammatory adipokines such as adiponectin,22 and harbor intrinsic cardiac ganglia and neuronal plexus that respond to ischemic stress.23 Notwithstanding these possible cardioprotective functions, excess epicardial fat may induce myocardial dysfunction through myocardial steatosis24 and/or infiltration between myocardial fibers and bundles.25 It may promote oxidative stress,26 inflammation,18 insulin resistance,27 diabetes mellitus type 2,28,29 and metabolic syndrome30 and predispose to coronary artery disease and cardiomyopathy.5,17,31 It is cross-sectionally associated with atrial fibrillation,32 which augments the risk of HF.33,34 Accompanying factors such as essential hypertension,35 ventricular hypertrophy,8 diastolic dysfunction,36 and hemodynamic alteration37 may precipitate the syndrome of HF38 and result in an obese-HFpEF phenotype.39
The cardiovascular implications of paracardial fat are not well-studied. Some studies indicate that paracardial fat is better correlated with cardiometabolic risk factors than epicardial fat.9,40 Perhaps the accumulating epidemiologic association between pericardial fat (a composite of epicardial and paracardial fat) and coronary atherosclerosis,41 obstructive coronary artery disease,42 incident myocardial infarction,43 and newly diagnosed HF1-3 will provide the needed impetus for the research community to direct attention toward examining the physio-pathological connotations of paracardial fat on the heart.
References
- Kenchaiah S, Ding J, Carr JJ, et al. Pericardial fat and the risk of heart failure. J Am Coll Cardiol 2021;77:2638-52.
- Kenchaiah S, Budoff MJ, Ding J, Carr JJ, Bluemke DA. Reply: Prognostic role of pericardial fat on the incidence of heart failure. J Am Coll Cardiol 2021;78:e113-e115.
- Rao VN, Bush CG, Mongraw-Chaffin M, et al. Regional adiposity and risk of heart failure and mortality: the Jackson Heart Study. J Am Heart Assoc 2021;10:e020920.
- Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B 1989;94:225-32.
- Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007;153:907-17.
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536-43.
- Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol 1995;76:414-48.
- Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 2004;13:313-16.
- Sironi AM, Petz R, De Marchi D, et al. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabet Med 2012;29:622-27.
- Barber MC, Ward RJ, Richards SE, et al. Ovine adipose tissue monounsaturated fat content is correlated to depot-specific expression of the stearoyl-CoA desaturase gene. J Anim Sci 2000;78:62-68.
- Bambace C, Telesca M, Zoico E, et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc Pathol 2011;20:e153-56.
- Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 2009;94:3611-15.
- Cheung L, Gertow J, Werngren O, et al. Human mediastinal adipose tissue displays certain characteristics of brown fat. Nutr Diabetes 2013;3:e66.
- Truong MT, Erasmus JJ, Munden RF, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol 2004;183:1127-32.
- Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes 1990;14:1013-22.
- Pezeshkian M, Noori M, Najjarpour-Jabbari H, et al. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab Syndr Relat Disord 2009;7:125-31.
- Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev 2007;8:253-61.
- Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460-66.
- Keegan J, Gatehouse PD, Yang GZ, Firmin DN. Spiral phase velocity mapping of left and right coronary artery blood flow: correction for through-plane motion using selective fat-only excitation. J Magn Reson Imaging 2004;20:953-60.
- Vural B, Atalar F, Ciftci C, et al. Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc Pathol 2008;17:392-98.
- Prati F, Arbustini E, Labellarte A, et al. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J 2003;24:329-36.
- Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005;29:251-5.
- Huang MH, Ardell JL, Hanna BD, Wolf SG, Armour JA. Effects of transient coronary artery occlusion on canine intrinsic cardiac neuronal activity. Integr Physiol Behav Sci 1993;28:5-21.
- Laennec RTH. De l'auscultation médiate, ou, Traité du diagnostic des maladies des poumons et du coeur : fondé principalement sur ce nouveau moyen d'exploration. Paris: J.-A. Brosson, et J.-S. Chaudé, 1819.
- Smith HL, Willius FA. Adiposity of the heart - A clinical and pathologic study of one hundred and thirty-six obese patients. Arch Intern Med (Chic) 1933;52:911-31.
- Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 2010;299:H202-09.
- Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab 2005;90:6300-02.
- Li Y, Liu B, Li Y, et al. Epicardial fat tissue in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol 2019;18:3.
- Greulich S, Maxhera B, Vandenplas G, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012;126:2324-34.
- Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 2003;88:5163-68.
- Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 2015;11:363-71.
- Thanassoulis G, Massaro JM, Cury R, et al. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol 2010;55:2491-98.
- Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920-25.
- Jamaly S, Redfors B, Omerovic E, Carlsson L, Karason K. Prognostic significance of BMI after PCI treatment in ST-elevation myocardial infarction: a cohort study from the Swedish Coronary Angiography and Angioplasty Registry. Open Heart 2021;8:e001479.
- Austys D, Dobrovolskij A, Jablonskiene V, Dobrovolskij V, Valeviciene N, Stukas R. Epicardial adipose tissue accumulation and essential hypertension in non-obese adults. Medicina (Kaunas) 2019;55:456.
- Iacobellis G, Leonetti F, Singh N, Sharma AM. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol 2007;115:272-73.
- Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail 2020;8:657-66.
- Kawel-Boehm N, Kronmal R, Eng J, et al. Left ventricular mass at MRI and long-term risk of cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2019;293:107-14.
- Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6-19.
- Sicari R, Sironi AM, Petz R, et al. Pericardial rather than epicardial fat is a cardiometabolic risk marker: an MRI vs echo study. J Am Soc Echocardiogr 2011;24:1156-62.
- Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914-19.
- Zhou J, Chen Y, Zhang Y, et al. Epicardial fat volume improves the prediction of obstructive coronary artery disease above traditional risk factors and coronary calcium score. Circ Cardiovasc Imaging 2019;12:e008002.
- Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2009;90:499-504.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Atherosclerotic Disease (CAD/PAD), Atrial Fibrillation/Supraventricular Arrhythmias, Lipid Metabolism, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Interventions and Coronary Artery Disease, Interventions and Imaging, Interventions and Vascular Medicine, Computed Tomography, Nuclear Imaging, Hypertension, Stress, Pericardial Disease
Keywords: Coronary Artery Disease, Heart Failure, Adiponectin, C-Reactive Protein, Interleukin-6, Natriuretic Peptide, Brain, Cardiovascular Diseases, Multidetector Computed Tomography, Waist Circumference, Atrial Fibrillation, Cardiometabolic Risk Factors, Essential Hypertension, Ethnic Groups, Follow-Up Studies, Hypothermia, Insulin Resistance, Intra-Abdominal Fat, Non-alcoholic Fatty Liver Disease, Stroke Volume, Risk, Subcutaneous Fat, Abdominal, Waist-Hip Ratio, Pericardium, Myocardial Infarction, Atherosclerosis, Diabetes Mellitus, Type 2, Hospitalization, Biomarkers, Coronary Circulation, Longitudinal Studies, Models, Statistical, Anti-Inflammatory Agents, Oxidative Stress, Cardiomyopathies, Dyslipidemias, Inflammation, Fatty Acids, Hypertrophy, Myocardium, Phenotype, Ganglia, Obesity
< Back to Listings