The Next Wave of Anticoagulation: Results of PACIFIC-AF and the Future Role of Factor XIa Inhibition in Atrial Fibrillation
Quick Takes
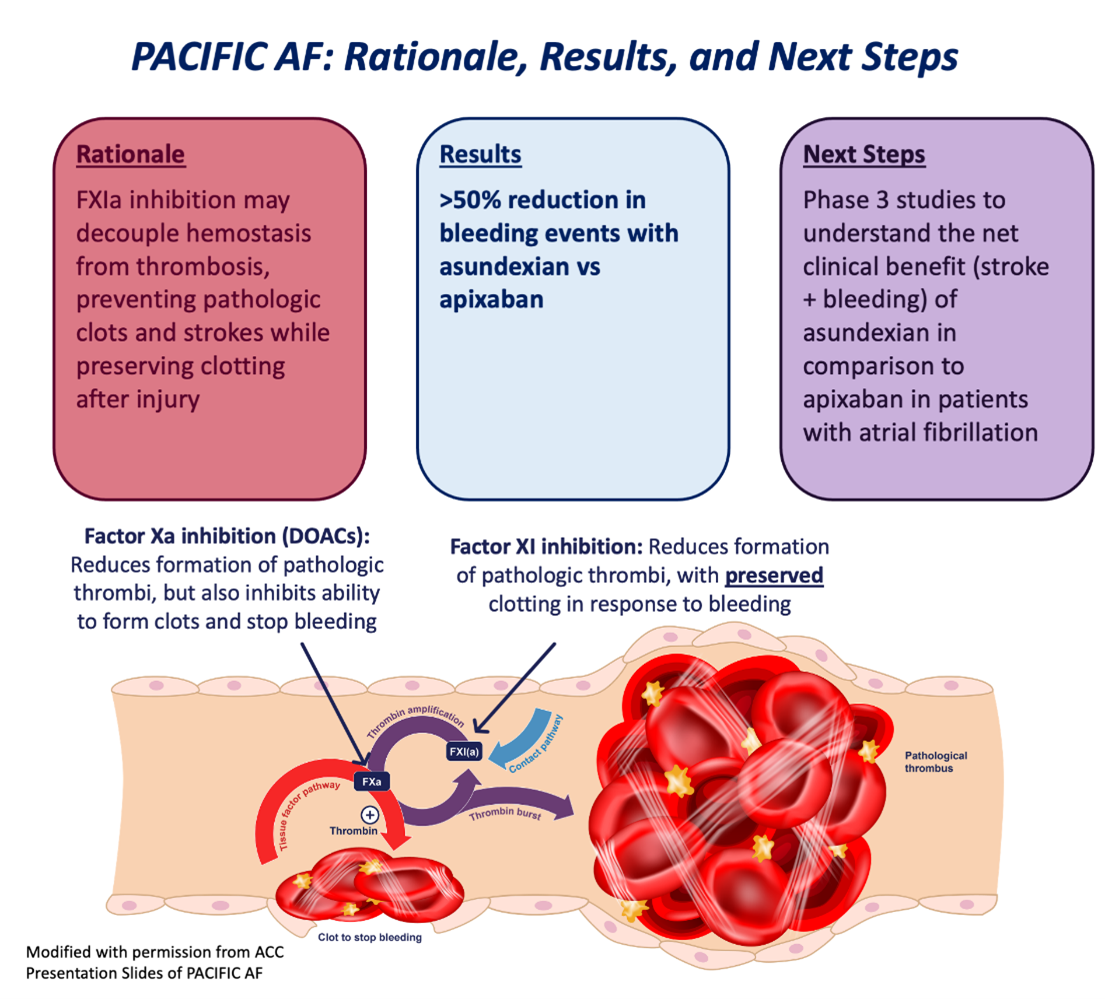
- Factor XIa inhibition may reduce stroke risk but preserve patients' ability to clot when bleeding.
- Phase 2 trial PACIFIC-AF showed a significant two-thirds reduction in significant bleeding with asundexian, an oral factor XIa inhibitor, in comparison to apixaban.
- Asundexian showed factor XI inhibition upwards of 90% with daily dosing in PACIFIC-AF.
- Factor XIa inhibition represents a promising strategy to reduce stroke risk while preserving a patient's ability to clot when bleeding.
Patients with atrial fibrillation (AF) are at an increased risk of stroke.1 For patients with a risk of stroke ≥2.2% per year (or CHA2DS2-VASc ≥2 for males and 3 for females), anticoagulation is recommended.2 Currently, direct oral anticoagulants (DOACs) are first-line treatment for the prevention of stroke in patients with AF, based on lower associated risk of stroke, mortality and intracranial hemorrhage in comparison to warfarin.3,4 However, patients on DOACs face a bleeding risk of 2.7-3.5% per year, which has likely contributed to the widespread underuse of anticoagulation in patients with AF.4,5
The increased risk of bleeding seen with most anticoagulation is determined by the mechanism of a given anticoagulation medication on coagulation cascade. For instance, targeting Xa (as with apixaban or rivaroxaban) can prevent formation of a pathologic thrombus by inhibiting the contact pathway, but also reduces the ability of a patient to form a clot in response to an injury via the tissue factor pathway (Figure 1). One strategy to combat the increased risk of bleeding seen with contemporary anticoagulation strategies is to target a clotting factor that exclusively acts on the contact pathway, with minimal impact on the tissue factor pathway. Mechanistically, at the right dose this should prevent the formation of pathologic thrombi while preserving a patient's ability to achieve hemostasis and to stop bleeding.
Figure 1
Factor XIa (FXIa) inhibition therefore offers an attractive strategy to accomplish this, and initial models suggest that this theory is biologically plausible: patients with genetic FXIa deficiency have lower rates of venous thromboembolism (VTE) and stroke than the general population,6 and mice with genetically inhibited FXIa activity show low rates of thrombosis without increased bleeding.7 Other studies assessing FXIa inhibition in patients following orthopedic surgeries suggest that FXIa inhibition is both well-tolerated and effective at preventing venous thrombosis after surgery, with a low associated risk of bleeding.8,9
The recently released PACIFIC-AF trial therefore compared the safety of asundexian, an oral FXIa inhibitor at two doses (20mg and 50mg) to a DOAC (apixaban at clinically indicated doses) in patients with AF at increased risk for stroke.10 Asundexian was chosen for testing based on low levels (15%) of renal elimination, low interaction profile (it is neither inhibited nor induced by CYP3A4, not impacted by pH changes seen with other medications or certain foods) and observed tolerance in Phase 1 trials. The main objective of this phase 2 trial was to understand differences in bleeding for patients randomized to asundexian versus apixaban. Similar trials were conducted with asundexian added to antiplatelet therapy in patients following myocardial infarction and stroke, with results expected in late summer of 2023.11,12
Ultimately, 671 participants completed 12 weeks of study drug in PACIFIC-AF. Participants were similar to a typical AF population, with a mean age of 73.7 years (41% female) and with comorbidities including hypertension (89%), heart failure (44%), coronary artery disease (31%), diabetes (32%) and chronic kidney disease (29%). Overall, low rates of bleeding were observed in this 12-week trial, with no major bleeding events (defined as fatal, into a critical organ such as the brain, or requiring transfusion of 2+ units of blood). However, rates of bleeding severe enough to prompt urgent medical care occurred one-third as often for participants randomized to asundexian versus apixaban (HR 0.33, 95% CI 0.09-0.97). Participants taking asundexian were also more than 50% less likely to experience any bleeding at all in comparison to apixaban (HR 0.42, 95% 0.26-0.67). Interestingly, rates of bleeding were similar between 20mg and 50mg doses of asundexian, suggesting that asundexian dose did not drive bleeding risk, as has been observed with DOACs.4
Notably, the study leveraged a proprietary assay of FXIa inhibition, and found that patients who received the 20mg achieved over 80% FXIa inhibition at trough, and that patients taking 50mg of asundexian had near complete (92%) inhibition, even at trough. Only three strokes occurred over the limited duration of this trial, limiting any ability to determine effect on stroke reduction using this drug. The effectiveness of FXIa inhibition will therefore be a question of importance for future research. However, asundexian was well-tolerated overall.
These data support the potential role for FXIa inhibition in patients with AF and make this strategy a promising avenue for future study. Participants in PACIFIC-AF who took apixaban had rates of severe bleeding that were three times higher than those who took asundexian, supporting the theory that inhibition of FXIa preserves a patient's ability to clot in response to bleeding events. Higher dose of asundexian were associated with more robust FXIa inhibition, but not with higher bleeding rates as seen with the similar if not better effects with 50mg versus 20mg. Stroke reduction and overall net clinical benefit for patients with AF remain important unanswered questions, which will be addressed by the upcoming OCEAN trial. This trial is designed to extend the results of PACIFIC-AF, assessing bleeding events alongside stroke and net clinical benefit in a substantially larger patient population over several years. This trial will be well-positioned to assess the safety, efficacy, and net clinical benefit associated with asundexian versus apixaban, and therefore has the potential to be practice-changing for both patients and providers.
References
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol 2014;64:e1-76.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol 2019;74:104–32.
- Carnicelli AP, Hong H, Connolly SJ, et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation 2022;145:242–55.
- Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries. Circulation 2014;129:1568–76.
- Puy C, Rigg RA, McCarty OJT. The hemostatic role of factor XI. Thromb Res 2016;141 Suppl 2:S8–S11.
- Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol 2010;30:388–92.
- Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015;372:232–40.
- Weitz JI, Strony J, Ageno W, et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med 2021;385:2161–72.
- Piccini JP, Caso V, Connolly SJ, et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022;399:1383–90.
- National Library of Medicine (U.S.). (2020, March 11 - 2022, March 11). Multicenter, Randomized, Placebo Controlled, Double-blind, Parallel Group, Dose-finding Phase 2 Study to Evaluate the Efficacy and Safety of BAY 2433334 in Patients Following an Acute Myocardial Infarction. Identifier NCT04304534. https://clinicaltrials.gov/ct2/show/NCT04304534.
- National Library of Medicine (U.S.). (2020, March 25 - 2022, February 18). Multicenter, Randomized, Placebo-controlled, Double-blind, Parallel Group, Dose-finding Phase 2 Study to Evaluate Efficacy and Safety of BAY2433334 in Patients Following an Acute Non-cardioembolic Ischemic Stroke. Identifier NCT04304508. https://clinicaltrials.gov/ct2/show/NCT04304508.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Dyslipidemia, Heart Failure and Cardiomyopathies, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Atherosclerotic Disease (CAD/PAD), Anticoagulation Management and Atrial Fibrillation, Anticoagulation Management and Venothromboembolism, Atrial Fibrillation/Supraventricular Arrhythmias, Lipid Metabolism, Novel Agents, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Hypertension
Keywords: Anticoagulants, Atrial Fibrillation, Coronary Artery Disease, Cytochrome P-450 CYP3A, Diabetes Mellitus, Factor XIa, Heart Failure, Hemostasis, Hydrogen-Ion Concentration, Hypertension, Intracranial Hemorrhages, Myocardial Infarction, Orthopedic Procedures, Platelet Aggregation Inhibitors, Renal Elimination, Renal Insufficiency, Chronic, Rivaroxaban, Stroke, Thromboplastin, Thrombosis, Venous Thromboembolism, Venous Thrombosis, Warfarin
< Back to Listings