Never Above Suspicion: Getting to the Bottom of Cardiac Sarcoidosis Diagnosis and Treatment
Quick Takes
- Maintain a high level of suspicion for cardiac sarcoidosis in the setting of sustained ventricular arrhythmias/sudden cardiac death, advanced AV block, and unexplained heart failure.
- FDG-PET and CMR are central to both diagnosis and monitoring of cardiac sarcoidosis.
- An interdisciplinary care team approach is essential in the management of patients with sarcoidosis.
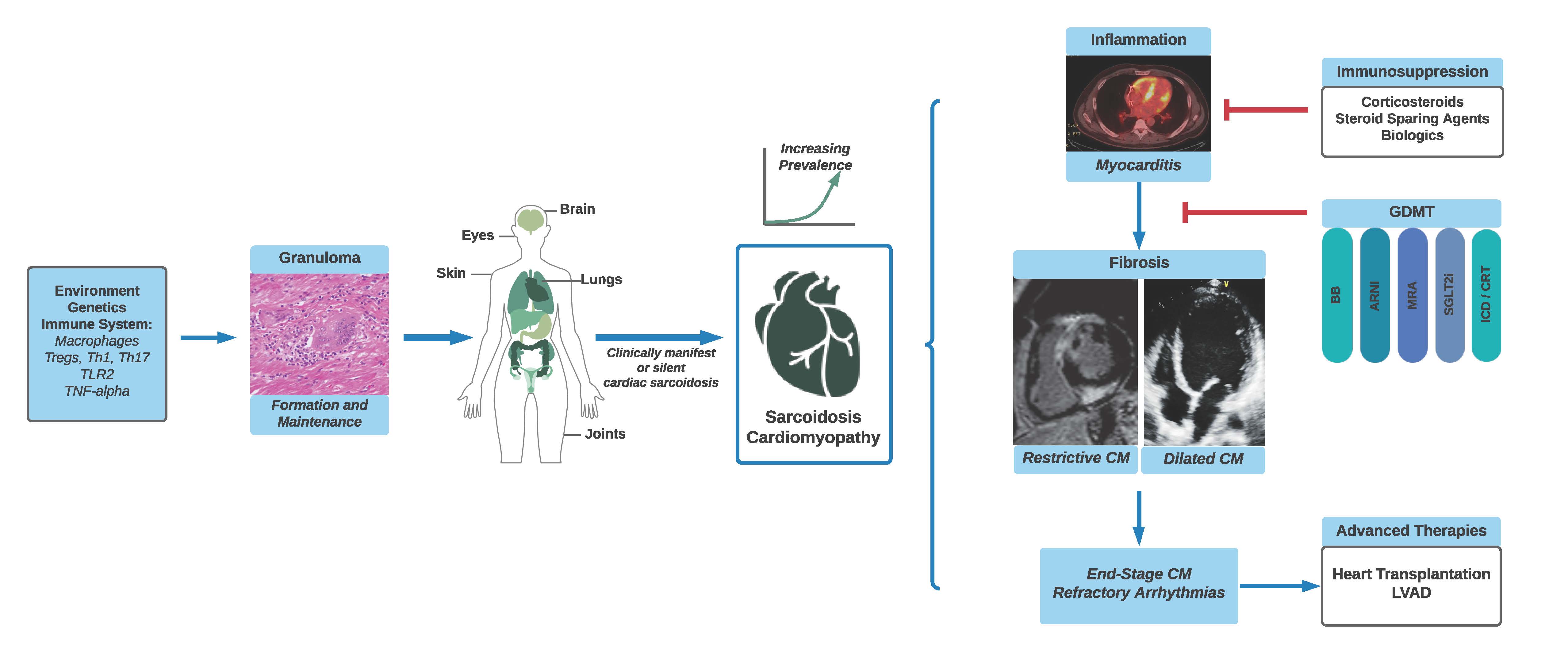
Sarcoidosis, an inflammatory granulomatous disease, develops through an interplay of genetic and environmental factors and affects multiple organs, including the heart (Figure 1). Though sarcoidosis is considered a rare disease, increased awareness and advancements in diagnostic techniques have led to a rise in the perceived prevalence of cardiac sarcoidosis (CS).1 This increased recognition has generated renewed interest in the field to improve diagnostic and therapeutic approaches.2
Figure 1
Presentation
In the United States (US), systemic sarcoidosis is more common in African Americans and females, though there is an increased incidence in those of Northern European descent.3 Among patients with systemic sarcoidosis, 5% have clinically manifest cardiac involvement presenting with heart failure (HF), ventricular arrhythmias and/or atrioventricular conduction disease whereas up to 40% may have asymptomatic or subclinical CS.4 A quarter of patients with CS may have isolated cardiac involvement, though the true prevalence is difficult to establish due to the insensitivity of current diagnostic criteria. Thus, early identification is imperative to halt progression of cardiac inflammation and resultant fibrosis. Screening for cardiac involvement of sarcoidosis with a detailed cardiac history, electrocardiogram (ECG), and echocardiogram should be considered in those with biopsy-proven extracardiac disease. In addition, suspicion should be high in the patients with sustained ventricular arrhythmias/sudden cardiac death, advanced atrioventricular block (especially < 60 years), and unexplained HF.
Diagnosis
Diagnostic criteria for CS include the Heart Rhythm Society (HRS)5 and the Japanese Circulation Society.6 The sensitivity of endomyocardial biopsy is less than 25% due to the patchy nature of the noncaseating granulomatous inflammation. Electrogram-guided endomyocardial biopsy increases the sensitivity to >50%, though is not widely performed.7 As a result, advanced cardiac imaging including 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) and cardiac magnetic resonance imaging (CMR) play a key role in diagnosis. Though the HRS criteria stipulate an endomyocardial or extracardiac biopsy consistent with sarcoidosis is necessary for a diagnosis of CS, the most recent iteration of the Japanese criteria allows for the diagnosis of CS without histopathology, in addition to diagnosis of isolated CS. Unfortunately, without universally accepted diagnostic criteria, there continues to be diagnostic heterogeneity. Further, CS presentation and imaging findings may overlap with other causes of myocarditis or arrhythmogenic cardiomyopathies, including inherited forms, emphasizing the need for thorough diagnostic investigation.
Imaging
FDG-PET and CMR are central to both diagnosis and monitoring of CS. FDG-PET leverages the high metabolic activity of sarcoid granulomas, primarily consisting of epithelioid macrophages ̶ after a period of low carbohydrate dietary intake, FDG is taken up preferentially by the inflammatory cells. FDG-PET studies are often paired with resting perfusion imaging; a region of augmented FDG uptake and concomitant decreased perfusion is highly sensitive for CS. CMR, conversely, is primarily utilized to identify areas of fibrosis by late gadolinium enhancement, typically subepicardial and mid myocardial in the basal to mid septal and/or inferolateral wall of the left ventricle (LV). Quantification of LV myocardial scar burden can be helpful in prognostication for outcomes such as risk for sudden cardiac death, with studies suggesting poorer outcomes for those with scar burden as low as 8% or more.8 Single session hybrid CMR/FDG-PET is an emerging imaging technique for the diagnosis and characterization of CS activity.9
Treatment
There are no universal guidelines on the treatment of CS, beyond the premise that corticosteroids are effective first line therapy. Recent data suggest no benefit for prednisone doses >40mg daily. Studies describing clinical response to corticosteroids have reported variable results, with between 20% to almost 50% recovery rate for AV block.10,11 Improvement in LV function is also variable in studies, some suggesting that those with severe dysfunction get the most benefit11 while others suggesting that those with only mild-moderate dysfunction achieve the best response.10 The consensus is that prednisone dose should be tapered over 9-12 months, facilitated by the addition of a steroid sparing agent, most commonly methotrexate or mycophenolate mofetil. Data supporting their use are limited to small retrospective studies.12-14 The actively recruiting CHASM-CS trial, which is investigating the use of prednisone monotherapy (max 30mg daily) compared with methotrexate and low-dose prednisone, will add much-needed data to this area. Finally, biologics are increasingly employed for more advanced CS or in cases of corticosteroid intolerance. TNF-α inhibitors, infliximab, and adalimumab, appear to be effective in treating inflammation in CS without the LV function compromise previously described in HF patients enrolled in the ATTACH and RENEWAL trials.15,16 Rituximab, an anti-CD20 antibody, may also be a promising addition to the armamentarium, though studies are limited.17
Outcomes
Death in CS is due to progressive HF or sudden cardiac death. In those with clinically manifest CS, 5-year overall survival from symptom onset ranges 85-96%.1,18 A European study reported 5- and 10-year transplantation-free cardiac survival of 90% and 83% for all CS patients. However, CS patients with HF had a 10-year transplantation free survival of only 53%.11 The prognosis of patients with subclinical disease is controversial, some suggesting a benign course and others reporting significant risk for VT/death.4 Disparities in sarcoidosis span race, sex, and socioeconomic status. For example, Black Americans have more severe sarcoidosis with more organs involved at presentation than White Americans.19 Females experience higher organ involvement at diagnosis and higher rates of hospitalization. Low-income patients experience higher sarcoidosis- and steroid-related comorbidities and lower health-related quality of life compared with higher income individuals.20 In addition, costly SSA and biologics may be inaccessible resulting in suboptimal treatment regimens. The impact of these factors on outcomes in CS requires further study, though highlights the need for early diagnosis and improved access to care.
Interdisciplinary Approach
Given its multisystem nature and complexities of management, patients with sarcoidosis benefit from an interdisciplinary care team structure. For CS, a collaboration may be established between HF specialists, electrophysiologists, cardiac imagers, pulmonologists, rheumatologists, and additional organ specialists as indicated. Pharmacists play a critical role in safe prescribing of immunosuppression and avoiding polypharmacy. Nursing support allows close monitoring for patient symptoms, treatment side effects, and coordination of complex patient care plans. Support groups can help educate and connect patients and their caregivers, who often bear significant psychosocial burden of having a rare disease. Finally, given the knowledge gaps, limited expertise, and relatively rare prevalence of CS, interinstitutional collaboration will be key in moving the field forward.
References
- Ekström K, Lehtonen J, Nordenswan HK, et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J 2019;40:3121-28.
- Gilotra NA, Griffin JM, Pavlovic N, et al. Sarcoidosis-related cardiomyopathy: current knowledge, challenges, and future perspectives state-of-the-art review. J Card Fail 2022;28:113-32.
- Milman N, Selroos O. Pulmonary sarcoidosis in the Nordic countries 1950-1982: epidemiology and clinical picture. Sarcoidosis 1990;7:50-57.
- Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol 2016;68:411-21.
- Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305-23.
- Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol 2017;3:42-45.
- Liang JJ, Hebl VB, DeSimone CV, et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail 2014;2:466-73.
- Smedema JP, van Geuns RJ, Ector J, Heidbuchel H, Ainslie G, Crijns HJGM. Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcome in pulmonary sarcoidosis. ESC Heart Fail 2018;5:157-71.
- Greulich S, Gatidis S, Gräni C, et al. Hybrid cardiac magnetic resonance/fluorodeoxyglucose positron emission tomography to differentiate active from chronic cardiac sarcoidosis. JACC Cardiovasc Imaging 2022;15:445-56.
- Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol 2013;29:1034-41.
- Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 2015;131:624-32.
- Rosenthal DG, Parwani P, Murray TO, et al. Long-term corticosteroid-sparing immunosuppression for cardiac sarcoidosis. J Am Heart Assoc 2019;8:e010952.
- Griffin JM, Chasler J, Wand AL, et al. Management of cardiac sarcoidosis using mycophenolate mofetil as a steroid sparing gent. J Card Fail 2021;12:1348-58.
- Hamzeh N, Voelker A, Forssen A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med 2014;108:1663-69.
- Gilotra NA, Wand AL, Pillarisetty A, et al. Clinical and imaging response to tumor necrosis factor alpha inhibitors in treatment of cardiac sarcoidosis: a multicenter experience. J Card Fail 2021;27:83-91.
- Baker MC, Sheth K, Witteles R, Genovese MC, Shoor S, Simard JF. TNF-alpha inhibition for the treatment of cardiac sarcoidosis. Semin Arthritis Rheum 2020;50:546-52.
- Elwazir M, Krause ML, Bois JP, et al. Rituximab for the treatment of refractory cardiac sarcoidosis: a single-center experience. J Card Fail 2022;28:247-58.
- Zhou Y, Lower EE, Li HP, Costea A, Attari M, Baughman RP. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest 2017;151:139-48.
- Rabin DL, Thompson B, Brown KM, et al. Sarcoidosis: social predictors of severity at presentation. Eur Respir J 2004;24:601-08.
- Harper LJ, Gerke AK, Wang XF, et al. Income and other contributors to poor outcomes in U.S. patients with sarcoidosis. Am J Respir Crit Care Med 2020;201:955-64.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Dyslipidemia, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Lipid Metabolism, Novel Agents, Acute Heart Failure, Computed Tomography, Magnetic Resonance Imaging, Nuclear Imaging
Keywords: Fluorodeoxyglucose F18, Gadolinium, Retrospective Studies, Contrast Media, Adalimumab, Atrioventricular Block, Infliximab, Methotrexate, Mycophenolic Acid, Prednisone, Quality of Life, Rituximab, Ursidae, Prevalence, African Americans, Cicatrix, Consensus, Heart Ventricles, Myocarditis, Pharmacists, Polypharmacy, Pulmonologists, Rare Diseases, Rheumatologists, Sarcoidosis, Positron-Emission Tomography, Magnetic Resonance Imaging, Electrocardiography, Biopsy, Prognosis, Adrenal Cortex Hormones, Death, Sudden, Cardiac, Perfusion Imaging, Hospitalization, Health Services Accessibility, Early Diagnosis, Self-Help Groups, Heart Failure, Inflammation, Patient Care, Macrophages, Patient Care Team, Granuloma, Perfusion
< Back to Listings