A Deep Patient-Similarity Learning Framework For Age-Invariant Recognition of Diastolic Dysfunction in Elderly Patients
The prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing with the aging population.1,2 However, developing tools to diagnose left ventricular diastolic dysfunction (LVDD) as the underlying mechanism for HFpEF in the older adult population is difficult due to age-related physiological changes in cardiac function.3-5
Deep Learning Patient-Similarity Framework (DeepNN) is a data-driven tool that can explore the multivariate echocardiographic relationships of diastolic dysfunction to identify patients at risk of future HF events.6,7 We previously described how a DeepNN classifier can be used to characterize the severity of LVDD and identify a specific subgroup of patients with HFpEF who have elevated LV filling pressures, biomarkers of myocardial injury and stress, and adverse events and those who are more likely to respond to therapeutic interventions.7 In this study, we sought to assess whether the DeepNN that was developed in younger cohorts could also be applied to the older population to identify subsets of patients with a heightened risk of developing HF since the DeepNN may be capable of automatically adjusting for age-related changes in LVDD.
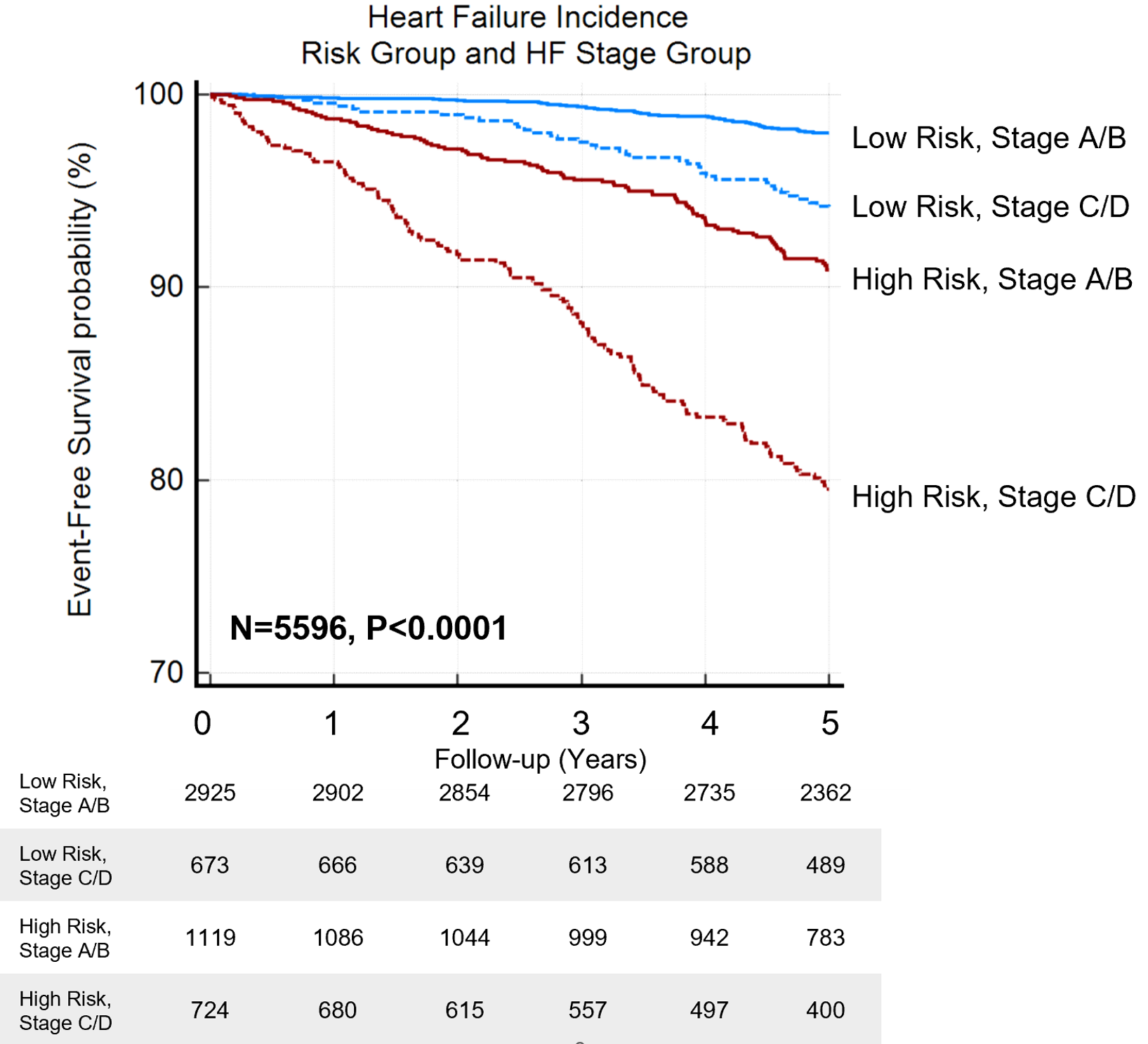
The database from Atherosclerosis Risk in the Community (ARIC) echocardiographic substudy was used to assess the DeepNN.8 The neural network reclassified patients with Stage A/B and C/D HF into high- and low-risk phenogroups where further analysis revealed a higher probability of incident HF in high-risk phenogroup in both stages (Kaplan Meier log-rank test p<0.0001 for all) (Figure 1).
Figure 1
In multivariate analyses, the DeepNN remained an independent predictor of incident HF in adjusted models with age, gender, race, and comorbidities for both Stage A/B (adjusted hazard ratio [HR], 95% confidence interval [CI] = 6.52[4.20-10.13], p<0.0001) and Stage C/D (adjusted HR [CI]=6.51[4.06-10.44], p<0.0001). In addition, there was a close correlation between the model-predicted age and chronological age (r=0.83, p<0.0001). Moreover, the DeepNN predictions showed incremental value over the 2016 American Society of Echocardiography (ASE) guideline grading, with an improvement in the continuous net reclassification index of >0.404 for both HF stage groups (p<0.0001 for all).
Our results highlight the utility of the deep learning framework in automatically extracting biological variables from echocardiographic data which obviates the need for inputting age or sex-related normal values while developing nomograms for clinical risk calculators. However, future studies would require prospectively validating these models in randomized clinical trials as a decision support tool before routine risk assessment can be implemented for early therapeutic interventions in clinical practice.
References
- Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106:3068-72.
- Forman DE, Fleg JL, Wenger NK. Cardiovascular disease in the elderly. In Zipes DP, Bonow RO, Mann DL, Tomaselli GF, Braunwald E, eds. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Elsevier; 2019.
- Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc 1988;63:137-46.
- Cheng S, Xanthakis V, Sullivan LM, et al. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation 2010;122:570-78.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321-60.
- Tokodi M, Shrestha S, Bianco C, et al. Interpatient similarities in cardiac function: a platform for personalized cardiovascular medicine. JACC Cardiovasc Imaging 2020;13:1119-32.
- Pandey A, Kagiyama N, Yanamala N, et al. Deep-learning models for the echocardiographic assessment of diastolic dysfunction. JACC Cardiovasc Imaging 2021;14:1887-1900.
- Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging 2014;7:173-81.
Clinical Topics: Heart Failure and Cardiomyopathies, Noninvasive Imaging, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Echocardiography/Ultrasound
Keywords: Stroke Volume, Heart Failure, Incidence, Prevalence, Confidence Intervals, Deep Learning, Nomograms, Reference Values, Echocardiography, Risk Assessment, Atherosclerosis, Biomarkers, Multivariate Analysis, Probability, Aging
< Back to Listings