Integrating Noninvasive Coronary Artery Plaque Imaging Into the Drug Development Pipeline
Quick Takes
- Noninvasive imaging techniques such as coronary artery calcium scoring can risk-stratify patients to help determine who may be most likely to benefit from novel pharmacotherapy.
- Advances in noninvasive coronary imaging techniques provide opportunities to integrate novel surrogate endpoints in the drug development pipeline.
- Surrogate imaging endpoints can provide an indication of drug efficacy before proceeding to phase 3 trials.
Commentary based on Figtree GA, Adamson PD, Antoniades C, et al. Noninvasive plaque imaging to accelerate coronary artery disease drug development. Circulation 2022;146:1712-27.
Introduction
Bringing novel therapies for coronary artery disease (CAD) to patients remains a major challenge because of the costs associated with trials and drug development.1 Traditionally, primary endpoints in trials evaluating novel therapies are clinical outcomes such as incident major adverse cardiovascular (CV) events or death. The incidence of such events is often low, requiring trials to have long follow-up periods and large sample sizes to be adequately powered. In other disease categories, surrogate endpoints are therefore often used; for example, systolic blood pressure has been validated as a surrogate for the clinical outcome of stroke.2 The Food and Drug Administration (FDA) has approved surrogate endpoints for >120 conditions, but there are no approved surrogates for CAD.2
Robust clinical outcome trials remain the "gold standard" for evaluating novel therapies for CAD, as highlighted by negative trial outcomes for drugs once thought to be promising. Recently, the PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients With Diabetes) trial failed to demonstrate a reduction in its primary endpoint despite post hoc analyses of other trials suggesting a benefit from fibrate therapy in individuals with elevated triglyceride (TG) levels and low high-density lipoprotein levels.3 However, advances in coronary imaging provide an opportunity to integrate surrogate endpoints earlier in the development pipeline before proceeding to phase 3 studies. Figtree et al. review various noninvasive coronary artery imaging modalities and discuss how these methods may be used to accelerate drug development.4
Noninvasive Imaging Modalities
Quantification of atherosclerosis by coronary artery calcium (CAC) scoring and computed tomography coronary angiography (CCTA) likely have the greatest access and scalability to be applied to drug development. Quantification of CAC improves risk estimation for atherosclerotic cardiovascular disease (ASCVD). Selective use in individuals without symptoms can be considered to refine estimated ASCVD risk and improve allocation of aspirin, statin, and nonstatin therapies. Absence of CAC (CAC = 0) is a powerful negative risk predictor indicating very low risk of ASCVD over the subsequent 5-10 years in persons who would otherwise be classified as intermediate risk by traditional risk factors.5 CCTA can assess coronary artery plaque burden, characterize individual plaque types, and measure coronary stenosis. Plaque characterization using CCTA can identify high-risk plaque characteristics such as low-attenuation plaque, and has the potential to serve as a comprehensive tool for the complete evaluation of coronary atherosclerosis because of its abilities to identify obstructive stenosis and measure atherosclerotic burden.6 Serial plaque assessment with CCTA over time can track coronary plaque and assess response to treatment or medication nonresponse via measurement of change/regression of low-attenuation and noncalcified plaque.6 Mapping of the perivascular fat attenuation index on CCTA enables measurement of coronary artery inflammation and was shown to enhance cardiac risk prediction in the CRISP-CT (Cardiovascular Risk Prediction using Computed Tomography) study.7
Nuclear imaging with positron emission tomography (PET)/computed tomography also has significant potential for characterizing atherosclerotic plaque. Radiotracers such as fluorine-18 (18F) fluorodeoxyglucose, gallium-68 DOTATATE, and coronary 18F fluoride can be used to detect high-risk coronary plaque and predict plaque progression. PET measures of atherosclerosis have been used in several trials to assess drug efficacy. However, PET imaging has considerations such as exposure to radiation, lower spatial resolution, and the instability of PET ligands that have limited its use more broadly and that make CCTA a more accessible and scalable modality. Similarly, magnetic resonance imaging can be used to identify high-risk coronary plaque but has been limited by inferior spatial resolution relative to computed tomography angiography (CTA) and by the availability of hardware and technical expertise (Table 1).
Table 1
| Modality | ||||
| CAC | CCTA | MRI | PET/CT | |
| Role | Screening of participants before enrollment in phase 3 trials | Use as an imaging endpoint in phase 2 studies Screening of participants before enrollment in phase 3 trials |
Identification of high-risk coronary plaque and stenosis | Identification of high-risk plaque |
| Pros | CAC burden robustly associated with ASCVD risk | Can assess plaque burden, coronary stenosis, and individual plaque characteristics | No ionizing radiation exposure | Possibility of specific molecular targeting in preclinical development (i.e., precision radiotracers) has been demonstrated in animal models |
| Cons | Cannot evaluate regression of noncalcified atherosclerosis | Requires IV contrast before medication to optimize image quality |
Low availability of specialized MRI hardware and lower spatial resolution than that of CCTA Requires IV contrast |
PET ligands unstable; higher exposure to radiation than with CAC and CCTA |
ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CCTA = coronary computed tomography angiography; CT = computed tomography; IV = intravenous; MRI = magnetic resonance imaging; PET = positron emission tomography.
Integration of Noninvasive Imaging in Trial Design
Noninvasive imaging may be used to identify distinct disease pathways that drive CAD and the effect of drugs on plaque progression, providing mechanistic insights on therapies in development. For example, the PARADIGM (Progression of Atherosclerotic Plaque DetermIned by Computed Tomographic Angiography Imaging) study examined the effect of statin therapy on serial CCTA scans. Statin therapy was associated with a lower volume of low-attenuation plaque and a higher volume of calcium-containing plaque on serial CCTA, supporting the concept that statin therapy transforms metabolically active plaque to a more stable plaque phenotype.8 The EVAPORATE (Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients With Elevated Triglycerides on Statin Therapy) study examined the mechanism of benefit of icosapent ethyl after the REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial) demonstrated a 32% reduction in CV events in patients with elevated TG levels. Icosapent ethyl was shown to cause significant regression of low-attenuation plaque volume (LAPV) compared with placebo.9 High-risk plaque characteristics such as a thin fibrous cap, lipid-rich composition, and large necrotic core can be identified on CCTA, which may allow for histopathological correlation with the molecular pathways driving CAD and plaque characterization to inform therapeutic targets.
A surrogate endpoint should be reproducible, be easily measurable, have a mechanistic biological basis, and have a direct correlate to a clinically important outcome.4 These criteria have been most consistently demonstrated in studies of statin therapy and PCSK9 inhibitors with imaging endpoints. A meta-analysis of 10 serial CCTA studies demonstrated the power of serial imaging using plaque measurements such as total plaque, noncalcified plaque, or LAPV as a primary endpoint in trials involving statin therapy.10 In all the studies, LAPV was decreased in participants receiving statin therapy. Use of surrogates such as LAPV in phase 2 trials may allow early demonstration of efficacy before proceeding to larger, costly phase 3 trials. The GOLDILOX (Efficacy and Safety of MEDI6570 in Patients With a History of Myocardial Infarction) trial, which is studying an antibody to lectin-like oxidized low-density lipoprotein receptor 1, and the PASSIVATE (PASSIvation of Vulnerable Plaque With AZD5718 in AcuTe Coronary syndromE) trial, which is studying a 5-lipooxygenase inhibitor, are in process and use plaque volume on serial CTA as the primary endpoint.4
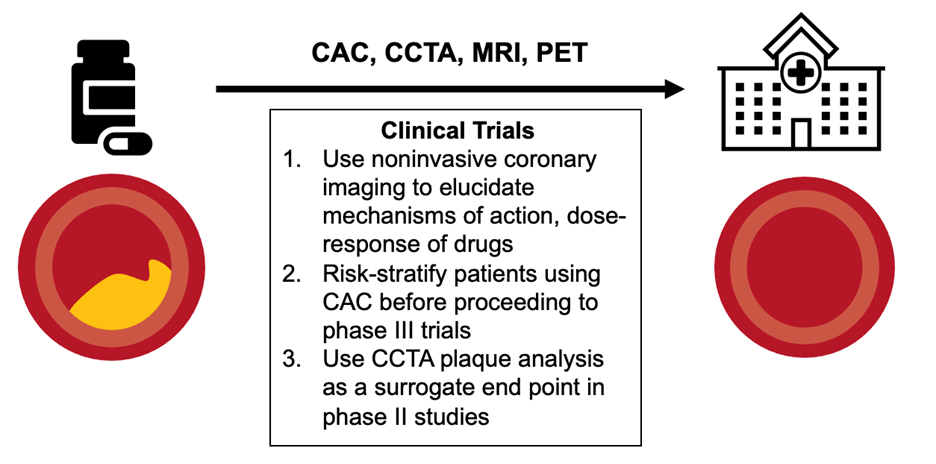
CAC and CCTA can be integrated at different points in the trial process. CCTA is likely more helpful in earlier-phase trials. Plaque regression may be used as a surrogate outcome to assess drug efficacy in phase 2 trials, whereas CAC and plaque analysis via CCTA has the power to aid in participant selection by identifying participants at high risk to be included in phase 3 trials, thereby reducing sample size by excluding individuals at low risk (Figure 1).
Figure 1
Translation of therapies for CAD to the clinic requires robust trials; however, noninvasive imaging can accelerate the process by: 1) allowing elucidation of pathophysiology, mechanisms of action, and dose response; 2) risk-stratifying patients before proceeding to phase 3 trials to reduce sample size; and 3) serving as a surrogate endpoint in phase 2 studies.
CAC = coronary artery calcium; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; MRI = magnetic resonance imaging; PET = positron emission tomography.
Conclusion
The integration of imaging endpoints can provide an earlier indication of drug efficacy and valuable insight into which treatments are most likely to reduce ASCVD outcomes. Moreover, noninvasive imaging can better risk-stratify groups to include individuals at higher risk who are most likely to benefit from novel treatment therapies, thereby reducing phase 3 trial sizes. Advances in noninvasive coronary imaging have the potential to transform the way future trials are conducted and to accelerate the translation of novel therapeutics.
References
- Figtree GA, Broadfoot K, Casadei B, et al. A call to action for new global approaches to cardiovascular disease drug solutions. Circulation 2021;144:159-69.
- Food and Drug Administration. Table of Surrogate Endpoints That Were the Basis of Drug Approval or Licensure (FDA website). 2022. Available at: https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure. Accessed 07/25/2023.
- Das Pradhan A, Glynn RJ, Fruchart JC, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med 2022;387:1923-34.
- Figtree GA, Adamson PD, Antoniades C, et al. Noninvasive plaque imaging to accelerate coronary artery disease drug development. Circulation 2022;146:1712-27.
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849-58.
- Abdelrahman KM, Chen MY, Dey AK, et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J Am Coll Cardiol 2020;76:1226-43.
- Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929-39.
- Lee SE, Chang HJ, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging 2018;11:1475-84.
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 2020;41:3925-32.
- Andelius L, Mortensen MB, Nørgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2018;19:850-8.
Clinical Topics: Acute Coronary Syndromes, Anticoagulation Management, Diabetes and Cardiometabolic Disease, Dyslipidemia, Invasive Cardiovascular Angiography and Intervention, Atherosclerotic Disease (CAD/PAD), ACS and Cardiac Biomarkers, Anticoagulation Management and ACS, Hypertriglyceridemia, Lipid Metabolism, Nonstatins, Novel Agents, Statins, Interventions and ACS, Interventions and Coronary Artery Disease, Interventions and Imaging, Angiography, Nuclear Imaging, Prevention, Noninvasive Imaging
Keywords: Plaque, Atherosclerotic, Coronary Artery Disease, Fluorodeoxyglucose F18, Calcium, Hydroxymethylglutaryl-CoA Reductase Inhibitors, PCSK9 protein, human, Cardiovascular Diseases, Proprotein Convertase 9, Fluorides, Acute Coronary Syndrome, Scavenger Receptors, Class E, Aspirin, Fibric Acids, Pharmaceutical Preparations, United States Food and Drug Administration, Lipoxygenase Inhibitors, Factor V, Risk Factors, Angiography, Atherosclerosis, Biomarkers, Phenotype, Inflammation, Triglycerides, Lipoproteins, Infarction
< Back to Listings