Statins For Cardioprotection From Anthracycline Cardiotoxicity
Quick Takes
- Statins have pleiotropic effects independent of their cholesterol-lowering properties, and there is ongoing interest in their potential as cardioprotective agents for patients with cancer receiving anthracycline therapy.
- Recent randomized controlled trials investigating the role of statins in preventing anthracycline-induced cardiotoxicity have yielded divergent results, highlighting the need for further research to identify the subgroups of patients who may benefit the most from up-front statin therapy.
- Although the available data do not conclusively support the universal use of cardioprotective statin therapy, the current guideline recommendation to consider statins for patients at high or very high risk, such as those receiving high-dose anthracyclines, seems appropriate.
Hydroxy-methyl-glutaryl coenzyme A reductase inhibitors, or statins, have pleiotropic effects that are independent of their cholesterol-lowering properties. Among these effects is the indirect inhibition of small Ras homologous GTPases, including Rac1, which is a key regulator of reduced nicotinamide-adenine dinucleotide phosphate oxidase and type II topoisomerase,1 both of which have been shown to play a significant role in the development of anthracycline-induced cardiomyopathy.2 Therefore, there has been considerable interest in evaluating whether statins are effective cardioprotective agents in patients with cancer exposed to anthracyclines.
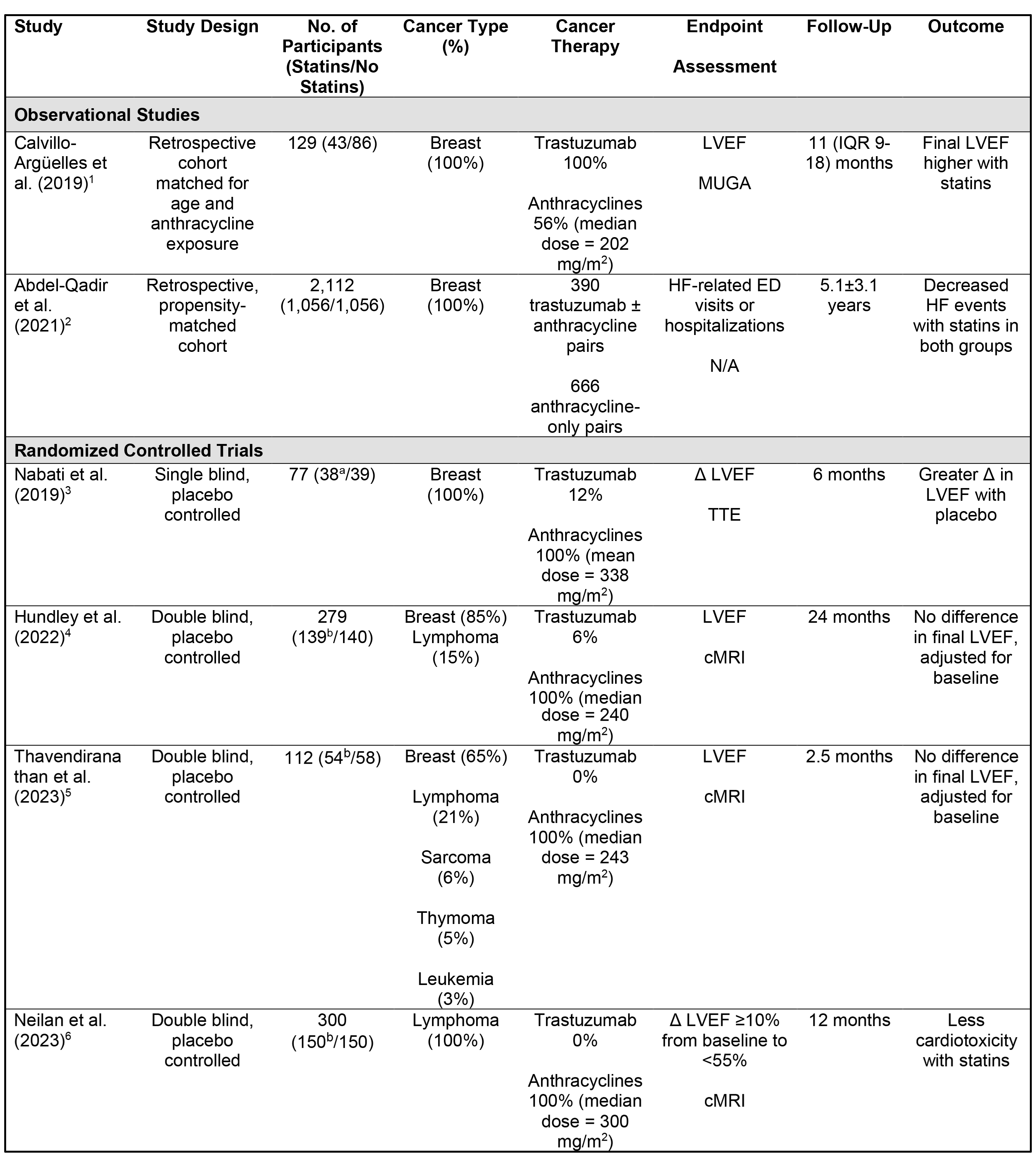
The 2022 European Society of Cardiology (ESC) guidelines give a class IIa recommendation for the use of statins for the primary prevention of cancer therapy-related cardiovascular toxicity (CTRCD) in patients at high and very high risk of developing CTRCD.3 This recommendation was based on retrospective observational studies and small randomized controlled trials (RCTs).4-6 However, within the previous year, there have been three randomized, double-blind, placebo-controlled trials investigating the cardioprotective role of statins in patients with cancer receiving anthracycline therapy, providing higher-quality data to support this recommendation (Table 1).
Table 1: Studies Evaluating the Cardioprotective Role of Statins in Patients Receiving Anthracycline Therapy
a Participants received rosuvastatin 20 mg daily
b Participants received atorvastatin 40 mg daily
Δ = change; cMRI = cardiac magnetic resonance imaging; ED = emergency department; HF = heart failure; IQR = interquartile range; LVEF = left ventricular ejection fraction; MUGA = multigated acquisition scan; TTE = transthoracic echocardiography.
Table References
- Calvillo-Argüelles O, Abdel-Qadir H, Michalowska M, et al. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can J Cardiol 2019;35:153-9.
- Abdel-Qadir H, Bobrowski D, Zhou L, et al. Statin exposure and risk of heart failure after anthracycline- or trastuzumab-based chemotherapy for early breast cancer: a propensity score‒matched cohort study. J Am Heart Assoc 2021;Jan 19:[ePub ahead of print].
- Nabati M, Janbabai G, Esmailian J, Yazdani J. Effect of rosuvastatin in preventing chemotherapy-induced cardiotoxicity in women with breast cancer: a randomized, single-blind, placebo-controlled trial. J Cardiovasc Pharmacol Ther 2019;24:233-41.
- Hundley WG, D'Agostino R Jr, Crotts T, et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid 2022;Aug 18:[ePub ahead of print].
- Thavendiranathan P, Houbois C, Marwick TH, et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur Heart J Cardiovasc Pharmacother 2023;Apr 29:[ePub ahead of print].
- Neilan TG, Quinaglia T, Onoue T, et al. Atorvastatin for anthracycline-associated cardiac dysfunction: the STOP-CA randomized clinical trial. JAMA 2023;330:528-36.
The PREVENT (Preventing Anthracycline Cardiovascular Toxicity with Statins) trial investigated the efficacy of atorvastatin 40 mg daily, started before anthracycline treatment and continued for 24 months, in 279 patients with breast cancer or lymphoma (median anthracycline dose 240 mg/m2). The primary endpoint of this study was the difference in 24-month left ventricular ejection fraction (LVEF), measured by cardiac magnetic resonance imaging (cMRI), between the placebo and treatment groups, adjusted for pretreatment LVEF.7 LVEF declined from 61.7±5.5% at baseline to 57.4±6.8% at 24 months with placebo and from 62.6±6.4% to 57.7±5.6% with atorvastatin. No significant difference in LVEF at 24 months was observed between the placebo and statin groups (-0.08 percentage points; p = 0.93). The secondary endpoint of CTRCD, defined as an absolute decline in LVEF of >10% from baseline to <50%, was observed in seven patients receiving placebo and four patients receiving atorvastatin.
The SPARE-HF (Statins for the Primary Prevention of Heart Failure in Patients Receiving Anthracycline Pilot Study) was a smaller (n = 112) randomized, placebo-controlled study that focused on patients at increased risk of CTRCD as defined by the American Society of Clinical Oncology (ASCO).8 The authors investigated the efficacy of atorvastatin 40 mg daily, administered during anthracycline treatment in a combined population of patients with breast, lymphoma, sarcoma, thymoma, and leukemia receiving a median anthracycline dose of 243 mg/m2. The primary endpoint was the difference in cMRI-measured LVEF at the end of anthracycline treatment between the placebo and statin groups. LVEF declined from 59.2±6.6% to 55.9±7.4% with placebo and from 60.2±5.4% to 57.3±5.8% with atorvastatin. Again, no difference in LVEF decline was observed between the placebo and statin groups (0.79%; p = 0.34). The secondary endpoint of CTRCD, defined as an absolute decline in LVEF of >10% from baseline to <53%, was observed in two patients in each group.
The STOP-CA (Statins to Prevent the Cardiotoxicity of Anthracyclines) trial investigated the efficacy of atorvastatin 40 mg daily, started before anthracycline treatment and continued for 12 months, in 300 patients with lymphoma treated with a median anthracycline dose of 300 mg/m2. The primary endpoint of this study was the proportion of patients who experienced CTRCD, defined as an absolute decline in cMRI-measured LVEF of ≥10% from baseline to <55% at 12 months. In this trial, patients treated with atorvastatin had a significantly lower incidence of CTRCD than did those who received placebo (9% vs. 22%; p = 0.002). Overall, LVEF declined significantly more after 12 months in the placebo group (62.5% to 57.1%) than in the atorvastatin group (62.9% to 58.8%), with an absolute difference of 1.3% (p = 0.029).
There are several explanations for the divergent results from these three RCTs. First, it is possible that only patients receiving high-dose anthracyclines derive benefit from up-front statin therapy. That anthracyclines cause a dose-dependent cardiotoxicity that increases in incidence at cumulative doses >250 mg/m2 is well known. The median dose of anthracyclines was higher in the STOP-CA trial than in the other two RCTs (300 mg/m2 vs. 240 mg/m2). Second, the PREVENT and SPARE-HF trials may have been underpowered to detect a difference in LVEF with statin therapy relative to placebo because of the high drop-out rate (36%) in the PREVENT trial and the small sample size (n = 112) of the SPARE-HF trial compared with the size (n = 300) and completion rate (>95%) of the STOP-CA trial. Third, the background prevalence of other cardioprotective therapies, such as renin-angiotensin-aldosterone antagonists and beta-blockers, differed between the trials and may have attenuated the observed effect of statins. Fourth, the duration of statin therapy and follow-up varied between the studies and may have influenced the results. For instance, both the SPARE-HF and STOP-CA trials included patients at increased risk of developing CTRCD. However, outcomes were evaluated after a median duration of 72 days in the SPARE-HF trial versus 12 months in the STOP-CA trial. Given that CTRCD can occur months after completion of anthracycline therapy, the shorter duration of follow-up in the SPARE-HF trial may have resulted in underdetection of CTRCD, and consequently of the benefits of statin therapy.
From a clinical perspective, the available data, albeit encouraging, do not conclusively support the universal use of statins as cardioprotective agents for anthracycline cardiotoxicity. Even though the STOP-CA trial achieved its primary endpoint, the absolute difference in LVEF at 12 months between patients randomized to statins versus placebo was only 1.3%. Additionally, there was no difference in the rate of clinical heart failure between the study groups. Therefore, the current ESC class IIa recommendation to consider statin therapy for cardioprotection in patients at high or very high risk seems appropriate.
Additionally, nonadherence to statins due to perceived adverse effects is common even among patients with coronary artery disease. Importantly, all three RCTs reported no increase in hepatotoxicity, myositis, and myopathy rates with statins. Despite this safety profile, universal prescription of statins with anthracycline treatment is unlikely to be accepted by patients without conclusive data supporting its efficacy. Further research is needed to determine which subgroups of patients with cancer will derive the greatest benefit from statin therapy, what the optimal timing should be, and what the duration of treatment should be.
References
- Henninger C, Fritz G. Statins in anthracycline-induced cardiotoxicity: Rac and Rho, and the heartbreakers. Cell Death Dis 2017;Jan 19:[ePub ahead of print].
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 2018;104:971-7.
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229-361.
- Nabati M, Janbabai G, Esmailian J, Yazdani J. Effect of rosuvastatin in preventing chemotherapy-induced cardiotoxicity in women with breast cancer: a randomized, single-blind, placebo-controlled trial. J Cardiovasc Pharmacol Ther 2019;24:233-41.
- Calvillo-Argüelles O, Abdel-Qadir H, Michalowska M, et al. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can J Cardiol 2019;35:153-9.
- Abdel-Qadir H, Bobrowski D, Zhou L, et al. Statin exposure and risk of heart failure after anthracycline- or trastuzumab-based chemotherapy for early breast cancer: a propensity score‒matched cohort study. J Am Heart Assoc 2021;Jan 19:[ePub ahead of print].
- Hundley WG, D'Agostino R Jr, Crotts T, et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid 2022;Aug 18:[ePub ahead of print].
- Thavendiranathan P, Houbois C, Marwick TH, et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur Heart J Cardiovasc Pharmacother 2023;Apr 29:[ePub ahead of print].
Clinical Topics: Cardio-Oncology, Dyslipidemia, Atherosclerotic Disease (CAD/PAD), Lipid Metabolism, Nonstatins, Novel Agents, Statins
Keywords: Atorvastatin, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Anthracyclines, Stroke Volume, Cardiotoxicity, Renin, Aldosterone, Breast Neoplasms, Thymoma, Coronary Artery Disease, Mineralocorticoid Receptor Antagonists, Ventricular Function, Left, Cardiotonic Agents, DNA Topoisomerases, Type II, Retrospective Studies, Angiotensins, ras Proteins, Monomeric GTP-Binding Proteins, Thymus Neoplasms, Sarcoma, Leukemia, Myositis, Medical Oncology, Chemical and Drug Induced Liver Injury, Oxidoreductases, Cardio-oncology
< Back to Listings