Have We All Gone MAD?
Quick Takes
- Mitral annulus disjunction (MAD) is an umbrella term encompassing different conditions.
- Isolated and limited MAD is a benign finding of the mitral annulus identified by advanced imaging modalities.
- In select patients with degenerative mitral valve disease marked MAD, there might be an arrhythmogenic marker, but larger studies are needed.
Tremendous enthusiasm about mitral annulus disjunction (MAD) emerged in 2018 when Dejgaard et al. showed that, in their cohort of patients with mitral valve prolapse (MVP), MAD was associated with an increased risk of malignant ventricular arrhythmias (VAs).1 However, the optimal treatment algorithm in the management of MAD as it pertains to the risk of sudden cardiac death (SCD) is diverse and remains incompletely understood. From 2018 onward, the number of peer-reviewed articles published on MAD soared by 700% as further evidence of the proarrhythmogenic role of MAD was gathered. The presence of MAD has been associated with myocardial fibrosis and premature death at autopsy,2 and with a high burden of ventricular ectopy in patients with Marfan syndrome.3 However, using advanced imaging modalities, MAD was found to be a common variant of the posterior mitral annulus and may occur even in the absence of MVP. The prevalence of MAD is not yet clearly determined and, in some study data, ranged from 76% to 96%.4,5 In fact, other studies' data have shown no association between MAD and SCD in patients with MVP at long-term follow-up.6
Several reasons underly this conflicting evidence. First, the heterogeneous definitions of MAD, which range widely between centers, hamper the comparison between studies (Table 1). MAD is generally defined as a systolic separation between the posterior atrial wall-leaflet junction and left ventricular (LV) wall.7 However, the prevalence of MAD can vary by 300% according to the imaging modality and diagnostic cutoff used to diagnose this condition. MAD is more frequently diagnosed when evaluated with advanced imaging modalities, which analyze with multiple imaging planes and better visualize the corresponding anatomy. Second, there is no consensus on a defined cutoff value for MAD.8 Because the prevalence of MAD varies so greatly, determining clinical management is hard.
Table 1: Extent and Prevalence of MAD in Different Study Cohorts
| Study Cohort | Imaging Modality | MAD Extent (mm) | MAD Prevalence (%) | |
| Carmo, 20109 | MVP | TTE | 7.4 ± 8.7 | 55 |
| Perazzolo Marra, 201610 | MVP | CMR | 4.6 (2.8-6.4) | 71 |
| Konda, 201711 | All-comer patients | TTE | – | 9 |
| Dejgaard, 20181 | MAD suspected on TTE | CMR | 3 (0-7) | 100 |
| Mantegazza, 20218 | MVP | TTE, TEE, CMR | 8 (7-10), 7 (5-8), 5 (4-7) | 17, 26, 42 |
| Guglielmo, 202112 | MVP | CMR | – | 23 |
| Essayagh, 202113 | MVP | TTE | 8 ± 3 | 31 |
| Demolder, 20213 | MFS | TTE | 6 (4-12) | 34 |
| Toh, 20214 | Structurally normal hearts | CCT | 3 (1.5-7) | 96 |
| Zugwitz, 20225 | Healthy volunteers | CMR | 3.4 ± 1.4 | 76 |
| Figliozzi, 20236 | MVP | CMR | 5.8 (3.4-7) | 68 |
CCT = cardiac computed tomography; CMR = cardiac magnetic resonance; MAD = mitral annulus disjunction; MFS = Marfan syndrome; MVP = mitral valve prolapse; TEE = transesophageal echocardiography; TTE = transthoracic echocardiography.
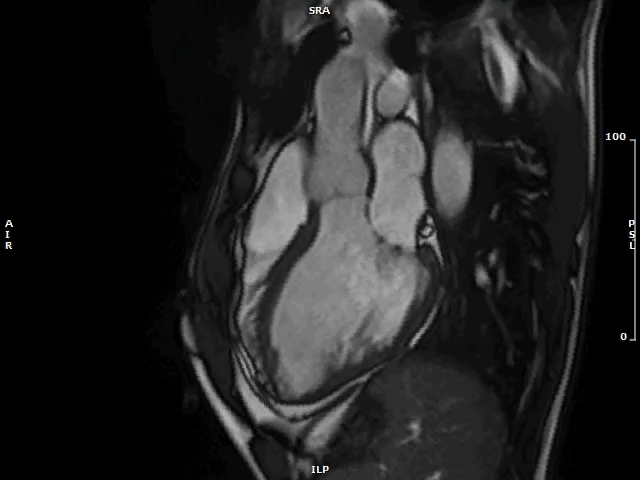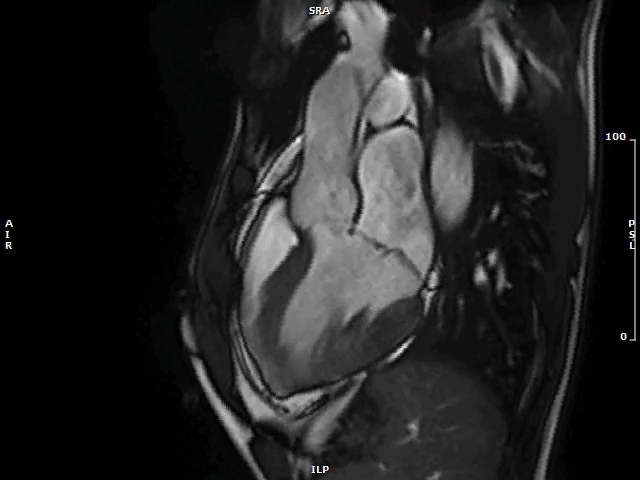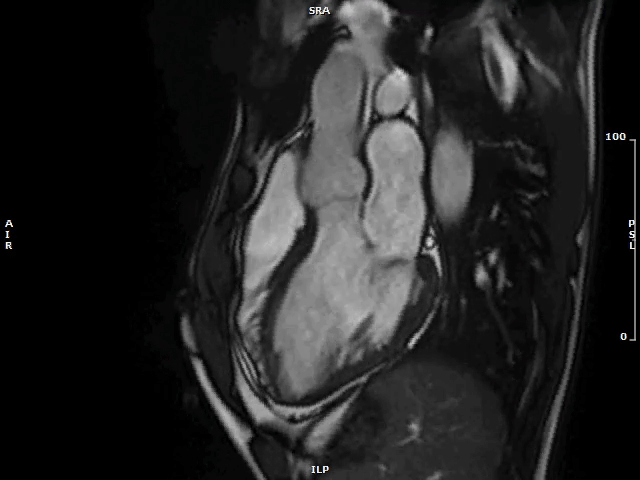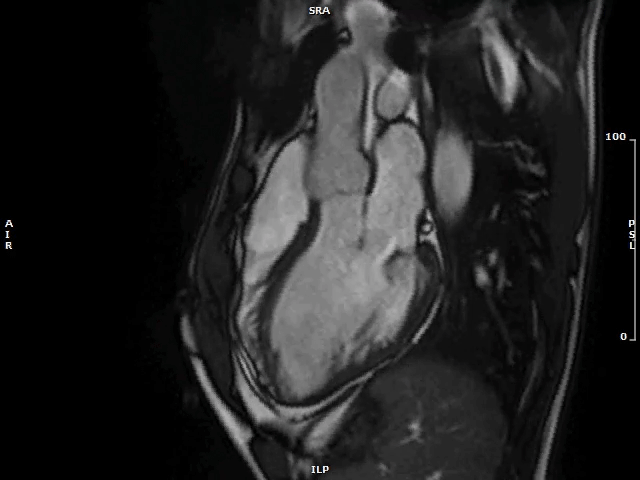
Cardiac computed tomography (CCT) and cardiac magnetic resonance (CMR), which have high spatial and contrast resolution, can unveil isolated MAD in healthy volunteers (Image 1).4,5 The imaging characteristics of this common MAD phenotype were defined as an excursion of ≥3 mm by CCT and CMR, with preferential location at the level of P1 and P3 scallops of the mitral valve (MV) and with minimal involvement of the P2 scallop. Although longitudinal validation is still needed, it is conceivable to consider such an imaging feature as innocent and benign with no clinical impact. It might represent a normal anatomical variant of the mitral annulus, which represents a subvalvular segment of the aorta-ventricular membrane deep into the mitral annulus, described by Dr. Friedrich Gustav Jakob Henle and other pathologists centuries ago.
Image 1: Isolated MAD on Cine cMRI
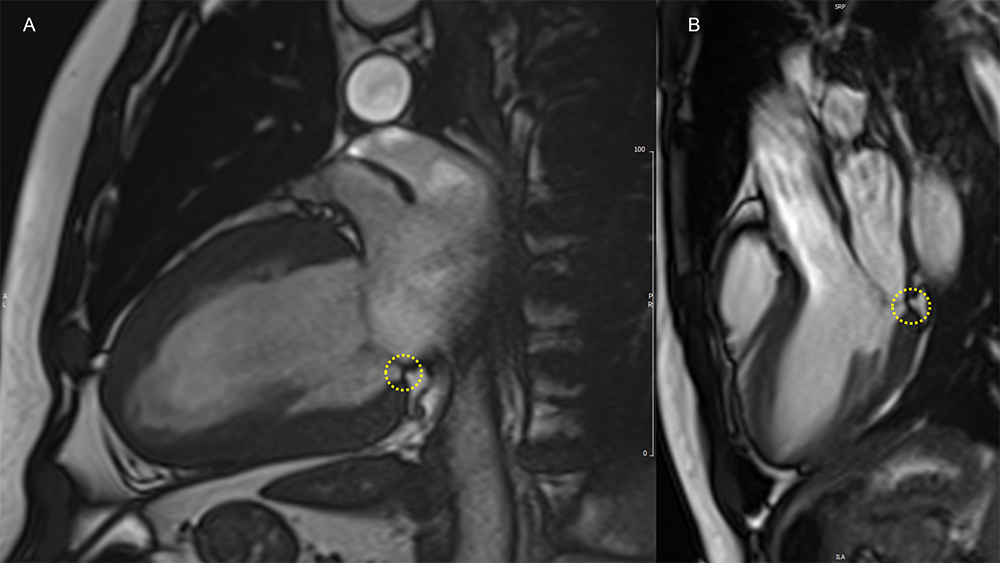
MAD (yellow circles) of limited extent is evident in a patient with hypertensive heart disease in the 2CH view (panel A), and in a healthy volunteer in the 3CH view (panel B).
2CH = two-chamber; 3CH = three-chamber; cMRI = cardiac magnetic resonance imaging; MAD = mitral annulus disjunction.
The association between MAD and patients with MVP is outdated and derives from pathological and transthoracic echocardiogram studies. The MAD phenotype in patients with MV apparatus degeneration differs in being more prevalent and extended, frequently involving the P2 scallop of the MV. Typically, it can be detected by the three-chamber or parasternal long-axis view; thus, the presence of MAD in these imaging views has been suggested to be pathological.7 However, early studies supporting the association of life-threatening arrhythmias and MAD in patients with MV degeneration were limited by cross-sectional design and selection bias, including patients with a high burden of malignant VAs.7 Recently, two studies assessed the longitudinal prognostic role of MAD in the setting of arrhythmic MVP assessed in the three-chamber view; the study data failed to demonstrate a significant role. In one echocardiography-based study,13 the presence of MAD did not lead to higher death rates at 10-year follow-up; however, it was associated with a combined arrhythmic endpoint. Nevertheless, the association persisted after mitral surgery and MAD resolution, raising concern about an independent arrhythmogenic role for MAD. In data from a CMR-based study, the presence of MAD was not associated with a composite arrhythmic outcome in contrast to myocardial fibrosis.6 However, particularly extended MAD might identify substrate for arrhythmogenic potentials.
The functional counterpart of extensive longitudinal MAD extent is a pronounced systolic outpouching and apical displacement of the atrium-valve leaflet junction with an exaggerated motion (curling) of the proximal LV inferolateral wall (Image 2; Video 1).7 The secondary forces transmitted to the LV wall and papillary muscles might bring about electrophysiological derangements encompassing a decrease of action potential duration and stretch-mediated early afterdepolarization,7 which subsequently may lead to the development of myocardial fibrosis through tension-mediated molecular pathways.14 In turn, myocardial fibrosis may act as a substrate for re-entry VAs, and unsurprisingly emerged as a key feature associated with malignant arrhythmias and adverse clinical outcomes in patients with MV apparatus degeneration.6,7 In line with this fascinating hypothesis, a good correlation between the longitudinal extent of MAD and the burden of myocardial fibrosis at CMR has been demonstrated,10 and an association between MADs >8.5 mm and VAs has been described in small cohorts of select patients.3,9
Image 2: MAD and MVP on Cine cMRI
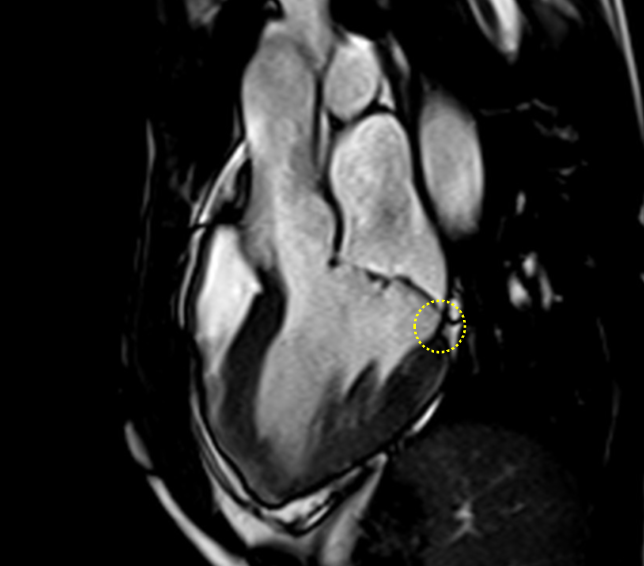
A great MAD (yellow circle) is evident in the 3CH view of a patient with MVP and history of palpitations and nonsustained VAs.
3CH = three-chamber; cMRI = cardiac magnetic resonance; MAD = mitral annulus disjunction; MVP = mitral valve prolapse; VA = ventricular arrhythmia.
Video 1: MAD Associated With Systolic Curling in a Patient With MVP
MAD = mitral annulus disjunction; MVP = mitral valve prolapse.
Overall, the available evidence refutes the sole presence of MAD as a proarrhythmic marker but does not exclude that particularly extended MADs might be arrhythmogenic by exerting a more significant stretch on the LV myocardial wall and papillary muscles, mechanically inducing ventricular ectopic beats and myocardial fibrosis (Figure 1). Larger prospective longitudinal studies on unselected consecutive patients are needed to search malignant longitudinal extents and locations of MAD predisposing to SCD. Before that, a uniform definition is mandatory.
Until then, MADness will reign.
Figure 1: The Clinical Significance of MAD
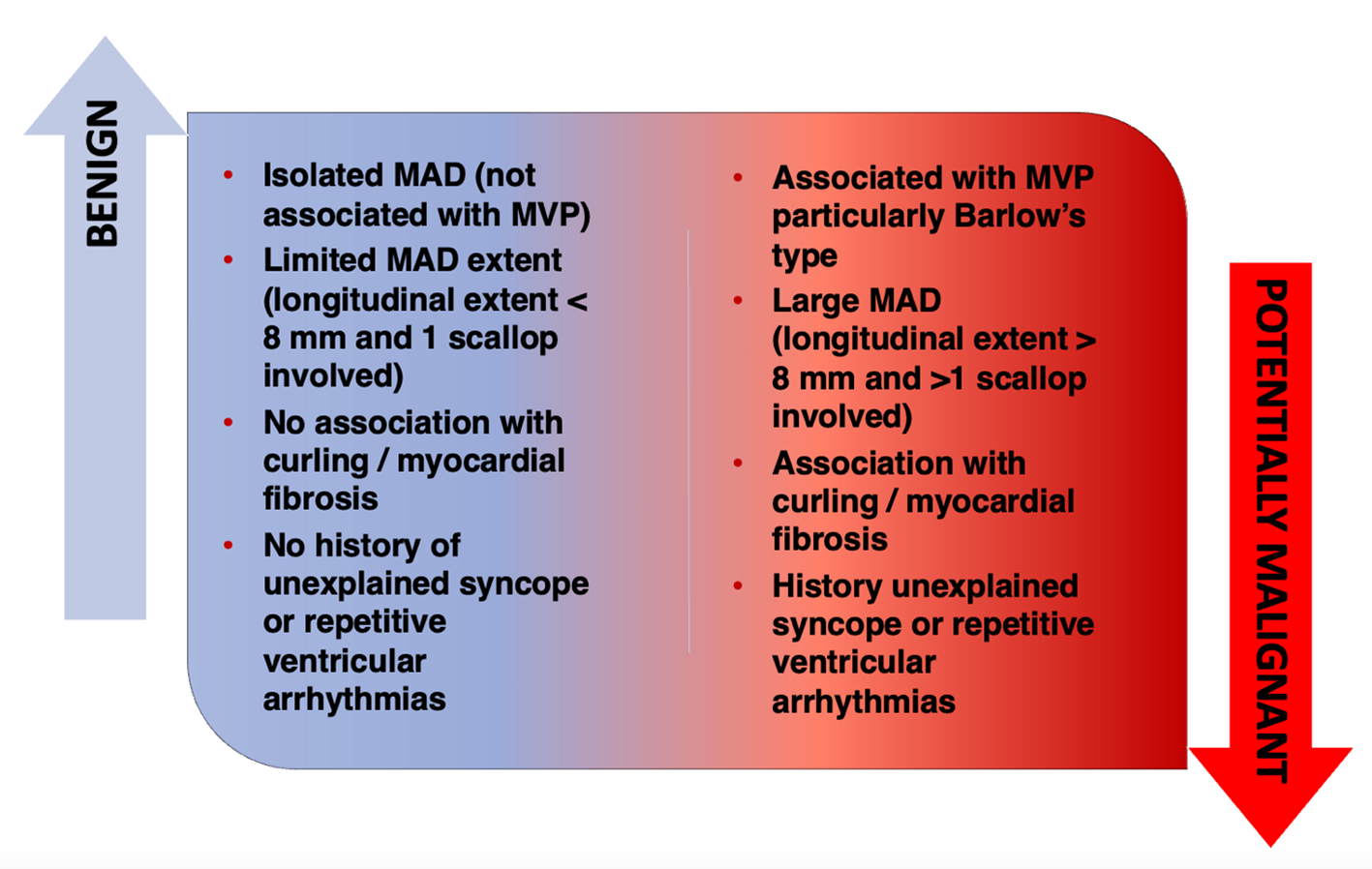
MAD = mitral annulus disjunction; MVP = mitral valve prolapse.
References
- Dejgaard LA, Skjølsvik ET, Lie ØH, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600-9.
- Zhou N, Zhao Q, Zeng X, et al. Association of mitral annular disjunction with premature cardiac mortality in a large series of autopsies. J Am Coll Cardiol 2021;77:102-4.
- Demolder A, Timmermans F, Duytschaever M, Muiño-Mosquera L, De Backer J. Association of mitral annular disjunction with cardiovascular outcomes among patients with Marfan syndrome. JAMA Cardiol 2021;6:1177-86.
- Toh H, Mori S, Izawa Y, et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: comprehensive three-dimensional analysis using cardiac computed tomography. Eur Heart J Cardiovasc Imaging 2021;22:614-22.
- Zugwitz D, Fung K, Aung N, et al. Mitral annular disjunction assessed using CMR imaging: insights from the UK Biobank population study. JACC Cardiovasc Imaging 2022;15:1856-66.
- Figliozzi S, Georgiopoulos G, Lopes PM, et al. Myocardial fibrosis at cardiac MRI helps predict adverse clinical outcome in patients with mitral valve prolapse. Radiology 2023;306:112-21.
- Basso C, Iliceto S, Thiene G, Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation 2019;140:952-64.
- Mantegazza V, Volpato V, Gripari P, et al. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021;107:25-32.
- Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound 2010;8:53.
- Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:[ePub ahead of print].
- Konda T, Tani T, Suganuma N, et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J Echocardiogr 2017;15:176-85.
- Guglielmo M, Fusini L, Muscogiuri G, et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur Radiol 2021;31:1100-9.
- Essayagh B, Sabbag A, Antoine 3, et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. JACC Cardiovasc Imaging 2021;14:2073-87.
- Morningstar JE, Gensemer C, Moore R, et al. Mitral valve prolapse induces regionalized myocardial fibrosis. J Am Heart Assoc 2021;10:[ePub ahead of print].
Clinical Topics: Arrhythmias and Clinical EP, Noninvasive Imaging, SCD/Ventricular Arrhythmias, Echocardiography/Ultrasound, Magnetic Resonance Imaging, Valvular Heart Disease
Keywords: Mitral Valve Prolapse, Death, Sudden, Cardiac, Magnetic Resonance Imaging, Echocardiography, Cardiac Imaging Techniques