18F Flurpiridaz PET MPI: New Horizons in Cardiac Imaging
Quick Takes
- Fluorine-18 flurpiridaz offers a long half-life, short positron range, and high first-pass myocardial extraction—enabling centralized production, exercise stress testing, superior spatial resolution, and quantification of myocardial blood flow.
- Compared with technetium-99m single-photon emission computed tomography, flurpiridaz positron emission tomography (PET) myocardial perfusion imaging demonstrates higher diagnostic performance, greater interpretive confidence, and improved image quality.
- Future directions include ongoing efforts to streamline protocols, expand availability, and conduct head-to-head comparisons with other currently used PET tracers.
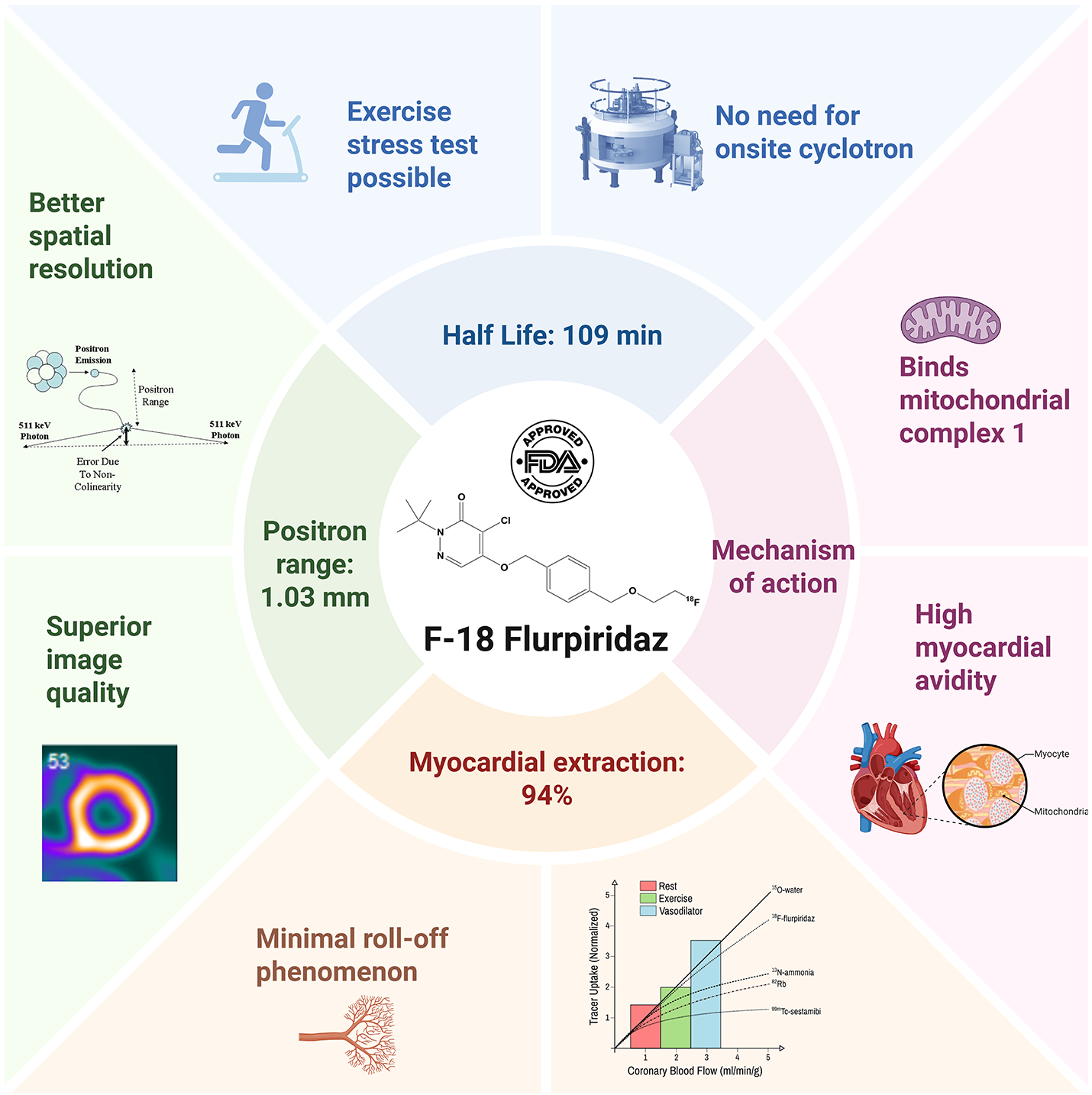
Fluorine-18 (18F) flurpiridaz is a radiotracer for positron emission tomography (PET) myocardial perfusion imaging (MPI) newly approved by the Food and Drug Administration (FDA), offering several advantages due to its favorable physical properties (Figure 1).
Figure 1: Physical Properties of Fluorine-18 Flurpiridaz
Created in BioRender. Alwan, M. (2025) https://BioRender.com/m1wscv7.
Why 18F Flurpiridaz?
First, flurpiridaz is an 18F-labeled structural analogue of the insecticide pyridine, which inhibits NADH: ubiquinone oxidoreductase, also known as mitochondrial complex I, an enzyme abundant in the mitochondria-rich myocardial tissue.1
Second, its relatively long physical half-life (109 min) allows for centralized production and unit-dose distribution, eliminating the need for an on-site cyclotron. This logistical advantage eliminates the barrier to PET MPI adoption in centers without advanced radiopharmacy infrastructure.2,3 Further, the long half-life of 18F allows for exercise stress testing, enabling simultaneous assessment of perfusion and physiologic response to exercise.1,2,4
Third, 18F has a positron range of 1.03 mm in water, which is shorter than that of rubidium-82 (8.6 mm), oxygen-15 labelled water (4.14 mm), and nitrogen-13 ammonia (2.53 mm), thereby improving spatial resolution and image quality.1,2
Fourth, flurpiridaz exhibits high first-pass myocardial extraction (94%) and a near-linear relationship with myocardial blood flow (MBF), with minimal roll-off at higher flows. This enables accurate MBF quantification and detection of mild perfusion defects.1
Current Evidence
The results of initial phase 1 studies demonstrated that 18F flurpiridaz is safe, with favorable dosimetry and biodistribution profiles.5
A phase 2 trial evaluated its safety and diagnostic performance against technetium-99m (Tc-99m) single-photon emission computed tomography (SPECT) MPI in 143 patients. Flurpiridaz was found to be safe and superior in terms of image quality, interpretive certainty, and overall coronary artery disease (CAD) diagnosis. PET sensitivity was significantly higher than that for SPECT (78.8% vs. 61.5%; p = 0.02), whereas specificity was comparable (76.5% vs. 73.5%).6
The first phase 3 trial included 755 patients to assess the diagnostic efficacy of flurpiridaz PET for detecting significant CAD (≥50% stenosis by quantitative invasive coronary angiography). Flurpiridaz PET demonstrated superior diagnostic performance by receiver operating characteristic (ROC) analysis compared with Tc-99m SPECT MPI, both in the overall population and in key subgroups, including women and patients with obesity (p < 0.001). Whereas sensitivity was significantly higher (71.9% vs. 53.7%; p < 0.001), specificity did not meet the prespecified noninferiority threshold (76.2% vs. 86.8%). Flurpiridaz PET also outperformed SPECT in image quality, diagnostic certainty, defect size, and radiation exposure reduction.7
A second phase 3 trial, the AURORA (International Study to Evaluate Diagnostic Efficacy of Flurpiridaz [18F] Injection PET MPI in the Detection of CAD), involved 578 patients with suspected CAD and confirmed the earlier findings. The results demonstrated higher specificity for flurpiridaz PET than for SPECT (63.8% vs. 61.7%; p = 0.0004). In addition, the area under the ROC curve was significantly greater with flurpiridaz PET than with SPECT in the overall population (0.8 vs. 0.68; p < 0.001), as well as among women and patients with obesity (p < 0.001 for both). Flurpiridaz PET met both the primary and secondary endpoints, with diagnostic sensitivity (80.3% vs. 68.7%; p = 0.0003) and specificity (63.8% vs. 61.7%; p = 0.0004) exceeding predefined thresholds, supporting its FDA approval.8
Image Characteristics With 18F Flurpiridaz
Due to the high spatial resolution of flurpiridaz, images are more susceptible to motion and misregistration artifacts, which should be carefully evaluated before interpretation. Additionally, subdiaphragmatic activity may be prominent but rarely limits interpretation. Imaging characteristics of normal variants with 18F are illustrated in Figure 2, panel A. Common findings in normal studies include apical thinning and uptake in the right ventricle and papillary muscles, typically more pronounced at rest than during stress.
In abnormal studies, perfusion defects and reversibility are usually more evident, and a more prominent transient ischemic dilation (TID) may be observed (Figure 2, panel B). However, no definitive TID cutoff has been established to date.
Figure 2: Perfusion Imaging Features With Normal and Abnormal 18F Flurpiridaz PET MPI Studies
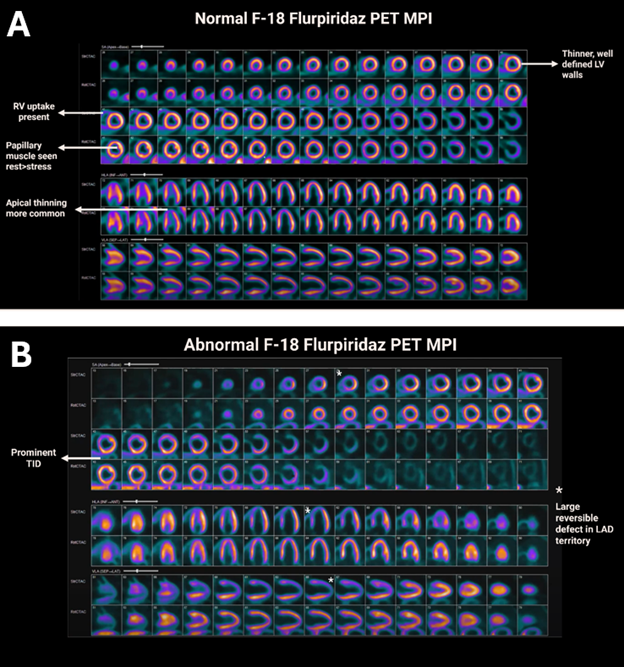
18F = fluorine-18; LAD = left anterior descending coronary artery; LV = left ventricle; MPI = myocardial perfusion imaging; PET = positron emission tomography; RV = right ventricle; TID = transient ischemic dilation.
Challenges and Future Directions
The current 18F protocol involves a 10-15 min rest acquisition, a 15-20 min delay for pharmacologic stress or a total of a 60 min delay for exercise stress,4 followed by a 10-15 min stress acquisition, resulting in a longer overall protocol duration than for currently available PET tracers.2 Efforts are underway to shorten the overall protocol duration by optimizing the stress-to-rest dose ratio rather than relying on delayed stress imaging to minimize interference from residual rest activity. One disadvantage of 18F is its slightly higher radiation exposure compared with that of currently available PET radiotracers, but the radiation exposure is lower than for SPECT.
Adoption of flurpiridaz has also been limited by high costs, restricted availability, and the need for specialized expertise.3,9 However, these barriers are being progressively addressed through ongoing training initiatives and educational workshops aimed at familiarizing technologists and clinicians with the tracer's kinetics, protocol requirements, and imaging characteristics. If clinical evidence supporting its diagnostic advantages continues to grow, wider clinical integration of 18F is anticipated.3
Future directions include ongoing trials directly comparing 18F flurpiridaz with currently available PET radiotracers to evaluate diagnostic performance.
References
- Maddahi J, Packard RR. Cardiac PET perfusion tracers: current status and future directions. Semin Nucl Med. 2014;44(5):333-343. doi:10.1053/j.semnuclmed.2014.06.011
- Maddahi J. 18F flurpiridaz PET MPI: imaging characteristics and clinical trials. J Nucl Cardiol. 2025;45S:102175. doi:10.1016/j.nuclcard.2025.102175
- Al-Mallah MH, Al Badarin F, Bengel F, et al. Potential worldwide impact of F-18 flurpiridaz positron emission tomography myocardial perfusion imaging. J Nucl Cardiol. 2025;45S:102167. doi:10.1016/j.nuclcard.2025.102167
- Bourque JM, Beanlands RSB, Berman DS, Chareonthaitawee P. F-18 flurpiridaz positron emission tomography myocardial perfusion imaging combined with treadmill exercise testing. J Nucl Cardiol. 2025;45S:102170. doi:10.1016/j.nuclcard.2025.102170
- Maddahi J, Bengel F, Czernin J, et al. Dosimetry, biodistribution, and safety of flurpiridaz F 18 in healthy subjects undergoing rest and exercise or pharmacological stress PET myocardial perfusion imaging. J Nucl Cardiol. 2019;26(6):2018-2030. doi:10.1007/s12350-018-01484-z
- Berman DS, Maddahi J, Tamarappoo BK, et al. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography. J Am Coll Cardiol. 2013;61(4):469-477. doi:10.1016/j.jacc.2012.11.022
- Maddahi J, Lazewatsky J, Udelson JE, et al. Phase-III clinical trial of fluorine-18 flurpiridaz positron emission tomography for evaluation of coronary artery disease. J Am Coll Cardiol. 2020;76(4):391-401. doi:10.1016/j.jacc.2020.05.063
- Maddahi J, Agostini D, Bateman TM, et al. Flurpiridaz F-18 PET myocardial perfusion imaging in patients with suspected coronary artery disease. J Am Coll Cardiol. 2023;82(16):1598-1610. doi:10.1016/j.jacc.2023.08.016
- Sanghani R, Al-Mallah MH, Thompson R. Challenges and strategies to enable access to cardiac positron emission tomography in different parts of the world: the North American perspective. J Nucl Cardiol. 2024;31:101790. doi:10.1016/j.nuclcard.2023.101790
Clinical Topics: Noninvasive Imaging, Atherosclerotic Disease (CAD/PAD), Computed Tomography, Nuclear Imaging, Stable Ischemic Heart Disease
Keywords: Myocardial Perfusion Imaging, Positron-Emission Tomography, Radioactive Tracers, Coronary Artery Disease