HELIOS-B Analyses: Vutrisiran Significantly Affects Biomarkers, Echocardiographic Parameters
Two post-hoc analyses of the HELIOS-B trial published Aug. 4 in JACC found that vutrisiran had significant effect on both biomarkers like NT-proBNP and troponin I levels and echocardiographic measures of biventricular systolic and diastolic function in patients with transthyretin amyloidosis cardiomyopathy (ATTR-CM). Furthermore, both these biomarkers and echocardiographic measures provide critical prognostic information.
In the double-blind, placebo-controlled HELIOS-B trial, investigators randomized 655 patients (mean age 76; 7% women; 88% wild-type transthyretin) with ATTR-CM and a clinical history of heart failure to subcutaneous injections of 25 mg vutrisiran or placebo every 12 weeks for up to 36 months.
During the trial, NT-proBNP and troponin I, both prespecified exploratory endpoints, were measured from blood at baseline and months 3, 6, 12, 18, 24 and 30; echocardiograms were performed at baseline and months 12, 18, 24 and 30.
Regarding biomarkers, results from the analysis by Mathew S. Maurer, MD, FACC, et al., showed that at 30 months, median changes from baseline for NT-proBNP and troponin I were 753 pg/mL and 9.7 pg/mL in the placebo arm compared to 118 pg/mL and –5.8 pg/mL in the vutrisiran arm. The geometric mean fold-change ratios (vutrisiran/placebo) were 0.68 (p<0.0001) for both NT-proBNP and troponin I.
Increases in NT-proBNP at six months were associated with higher risk of composite outcome of all-cause mortality and recurrent CV events (hospitalizations for CV causes or urgent visits for heart failure) up to 36 months and all-cause mortality up to 42 months; similarly, decreases in troponin I at six months were associated with a lower risk of the composite outcome (p<0.0001 for both).
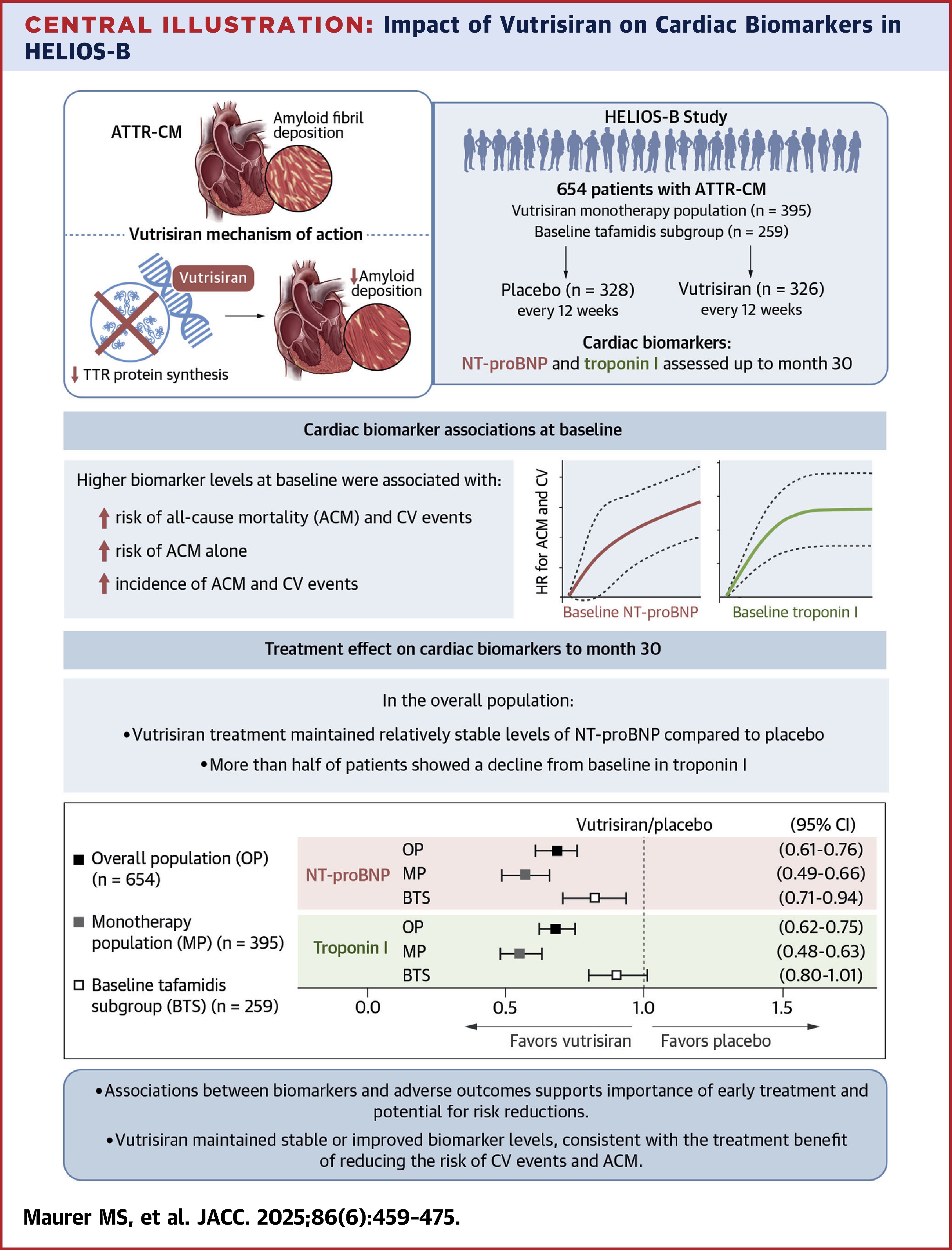
"Increasing NT-ProBNP at [six] months helps identify patients at highest cardiovascular risk at a relatively early time point," write Joshua D. Mitchell, MD, MSCI, FACC; Nicole Nakamatsu, MD; and Richard K. Cheng, MD, MSC, FACC; in an accompanying editorial comment. "Earlier identification may prompt additional heart failure therapy optimization and interventions or refinement of patient/clinician expectations on the patient's clinical course."
Regarding echocardiographic measurements, results from an analysis by Karola S. Jering, MD, et al., showed that at 18 months, vutrisiran reduced declines in both left and right ventricular systolic function (least squares mean difference for LVEF, 1.6%; absolute global longitudinal strain, 0.7%; tricuspid annular systolic myocardial velocity, 0.5 cm/s).
Additionally, left and right ventricular systolic and diastolic function were found to be independently associated with the primary outcome (HR per unit increase, LVEF, 0.90 per 5% increase; absolute global longitudinal strain, 0.92 per 1% increase; tricuspid annular systolic myocardial velocity, 0.94 per 1-cm/s increase; average E/e', 1.03 per 1-U increase), providing "important prognostic information in patients with ATTR-CM above and beyond the well-validated biomarker-based staging system," according to the analysis authors.
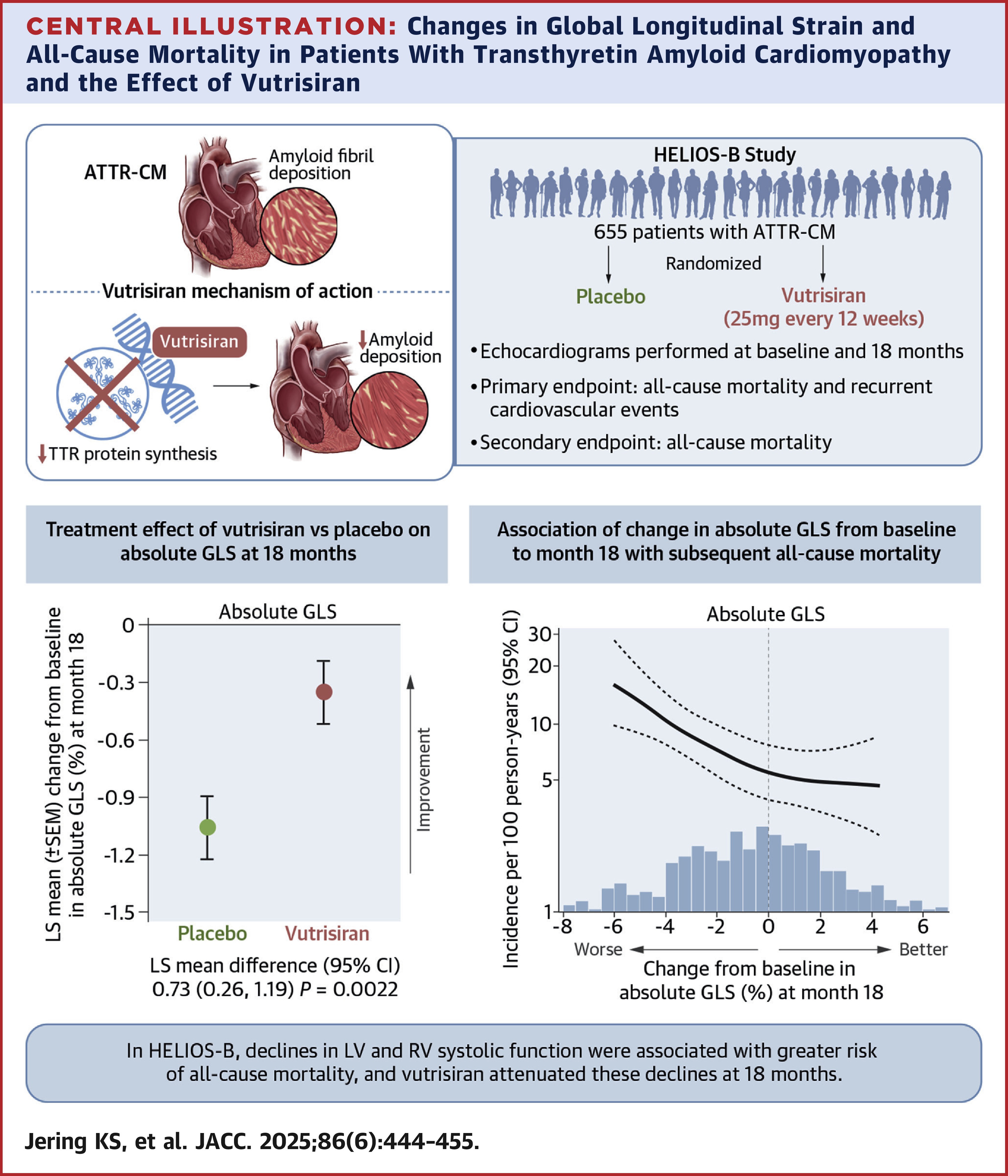
The analysis "offers hope of a future accurate indicator that may be used to counsel individuals with ATTR-CM regarding their prognosis," writes Michelle M. Kittleson, MD, PhD, FACC, in an accompanying editorial comment. "The association between changes in echocardiographic parameters with vutrisiran and subsequent clinical outcomes suggests a future role of such markers in assessing therapeutic efficacy and guiding the choice of available therapies."
Both Maurer and Kittleson were interviewed for an Aug. 4 article in the New York Times centered on this work and other ongoing research and advances for the diagnosis and treatment of ATTR-CM. "We might be living in an era where you will die with cardiac amyloidosis as opposed to from it," said Kittleson in the Times article.
Citations:
- Maurer, M, Berk, J, Damy, T, et al. Impact of Vutrisiran on Cardiac Biomarkers in Patients With Transthyretin Amyloidosis With Cardiomyopathy From HELIOS-B. J Am Coll Cardiol. 2025;86(6):459–475. doi: 10.1016/j.jacc.2025.04.055
- Jering, K, Fontana, M, Skali, H, et al. Effects of Vutrisiran on Cardiac Function and Outcomes in Patients With Transthyretin Amyloidosis With Cardiomyopathy.J Am Coll Cardiol.2025;86(6):444–455. doi: 10.1016/j.jacc.2025.06.022
Clinical Topics: Heart Failure and Cardiomyopathies, Noninvasive Imaging, Echocardiography/Ultrasound
Keywords: Echocardiography, Amyloidosis, Biomarkers, Troponin I
< Back to Listings
