Study Assesses Coronary Sinus Reducer Implantation in RA
Coronary sinus reducer (CSR) implantation may be safe and feasible, with promising antianginal efficacy in patients with refractory angina (RA), according to a meta-analysis published Aug. 11 in JACC: Cardiovascular Interventions. However, larger trials are needed to assess efficacy.
Utkarsh Ojha, MBBS, et al., conducted a meta-analysis of three double-blind, placebo-controlled randomized controlled trials (RCTs) comprised of 180 participants and 13 unblinded single-arm studies with 668 participants. Cohorts were predominantly men over 60 years of age, with obstructive coronary artery disease and high rates of prior revascularization and comorbidities. Median follow-up was six months for the RCTs and 7.2 months for the single arm studies. Outcomes included changes in Canadian Cardiovascular Society (CCS) and Seattle Angina Questionnaire (SAQ) classifications, as well as safety and other quality of life improvements.
Results showed that placebo-controlled rates were 26% (p<0.001) for ≥1 CCS class improvement and 17% (p=0.02) for ≥2 CCS class improvement, with a placebo-controlled exercise time change of 49.62 seconds (p=0.04), which the authors note as "modest but promising antianginal efficacy." However, SAQ estimates fell below the clinically relevant improvement threshold.
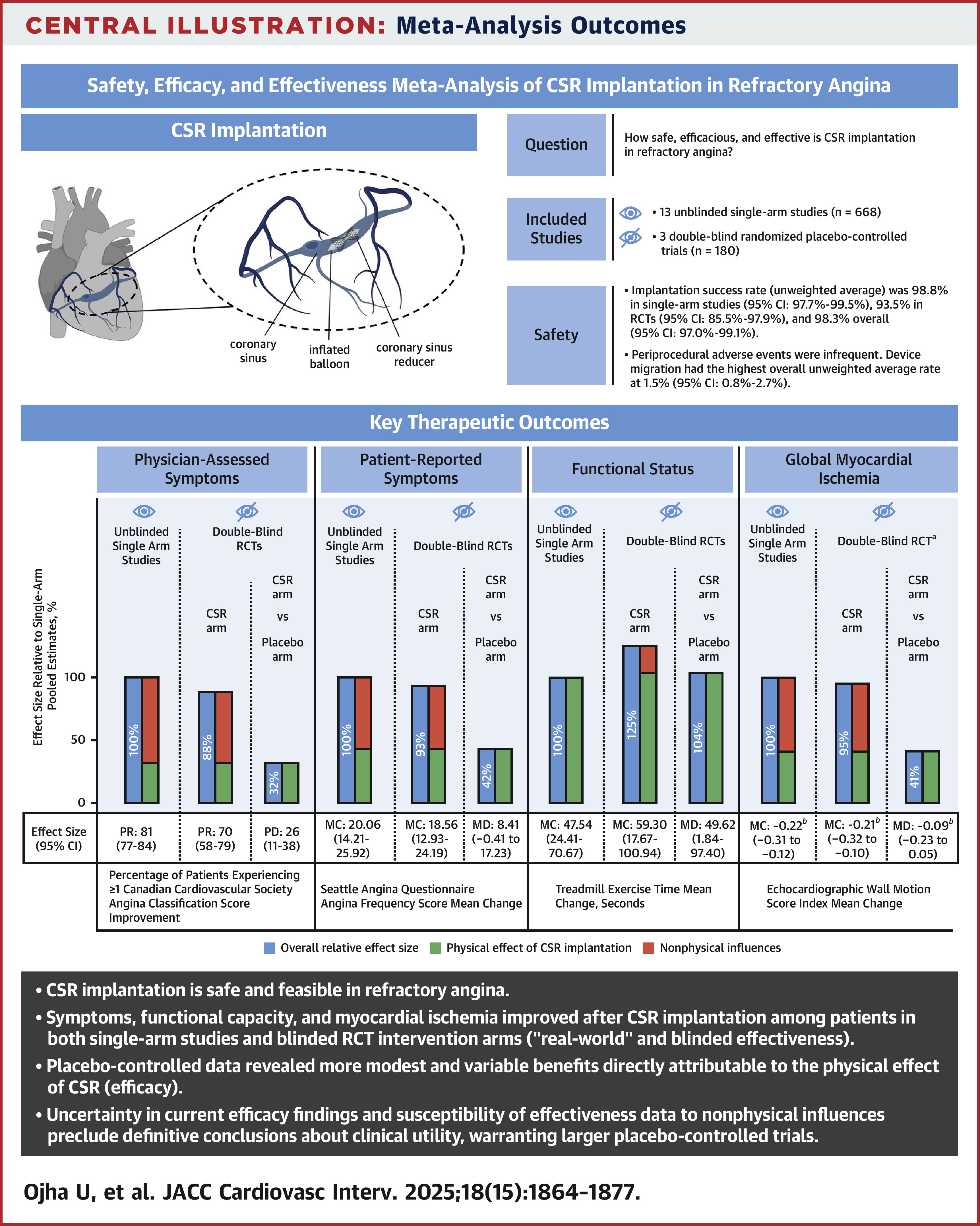
Procedural success rate was 98.3% with the most common complication being migration of the device, occurring in 1.5% of patients. There were four acute coronary syndrome events reported, but no periprocedural mortality or strokes.
"CSR demonstrates favorable safety across designs but carries non-negligible risks, including cardiac tamponade and device embolization, underscoring the need for robust efficacy evidence," write the authors.
"Although single-arm studies support substantial improvements in symptoms and quality of life following CSR implantation, double-blind placebo-controlled trials reveal smaller yet tangible benefits directly attributable to CSR implantation after accounting for factors such as the placebo effect and confounding," they add. "This highlights a notable gap between the observed effectiveness and actual efficacy of CSR in RA."
In an accompanying editorial comment, Michael Vavuranakis, MD, PhD, and Deepak L. Bhatt, MD, MPH, MBA, FACC, note that, "There may be a role for the CSR in future clinical practice, particularly for patients with [RA], as it might provide improvements in symptomatology and quality of life." However, they add that "health care systems should resist the pressure to approve and reimburse devices such as the CSR before robust data supporting utilization."
Keywords: Angina Pectoris, Coronary Sinus
< Back to Listings
