Beyond Diabetes: The Impact of Empagliflozin in Heart Failure with Reduced Ejection Fraction
Quick Takes
- This secondary analysis from the EMPEROR-Reduced trial shows that use of empagliflozin reduces the risk of CV death or HF hospitalization, as well as worsening renal function, across the glycemic spectrum in those with HF with reduced ejection fraction.
- This study, in addition to a previous one with dapagliflozin, confirms a benefit of SLGT2is in patients with HF with reduced ejection fraction regardless of diabetes status.
In 2008, based on increasing concern that some of the antihyperglycemic agents (AHAs) used to treat patients with diabetes might increase the risk of cardiovascular (CV) events, the Food and Drug Administration (FDA) mandated that all new AHAs would need to demonstrate CV safety.1 As a result, a number of large CV outcomes trials have taken place, and some of the newer agents have shown surprising benefits.2 The landmark trial EMPA-REG OUTCOME found that the sodium-glucose cotransporter 2 inhibitor (SGLT2i) empagliflozin was associated with a 38% reduction in CV death, as well as a 35% reduction in heart failure (HF) events in patients with type 2 diabetes mellitus (T2DM).3 A subsequent analysis of the trial additionally found a 39% reduction in worsening nephropathy, as well as fewer renal events such as initiation of renal replacement therapy or doubling of serum creatine with empagliflozin use.4 Similarly promising data was observed in trials evaluating other SLGT2i's such as canagliflozin5 and dapagliflozin.6
Mechanistically, the CV and renal benefits seen with SLGT2is cannot be attributed to lowering of glucose alone. EMPA-REG OUTCOME was designed to be conducted with glycemic equipoise, with comparable glucose control in the placebo and empagliflozin arms of the trial. The survival curves of the two arms of the trial separated almost immediately, with suggested mechanisms including enhanced natriuresis, improved energy metabolism, and reduced sympathetic tone.7 Ultimately, the CV and renal protection seen with SGLT2is is likely multifactorial, but does not appear to be related to its role as an AHA.
Therefore, there has been increasing interest in the utility of SGLT2i for patients without T2DM. This was first evaluated in the DAPA-HF trial, which found that dapagliflozin reduced the risk of HF hospitalization or CV death both for patients with HF with reduced ejection fraction (HFrEF) and with and without T2DM.8 These findings suggested that SGLT2is such as dapagliflozin may be more accurately described as cardiometabolic or heart failure drugs, rather than strictly as AHAs.
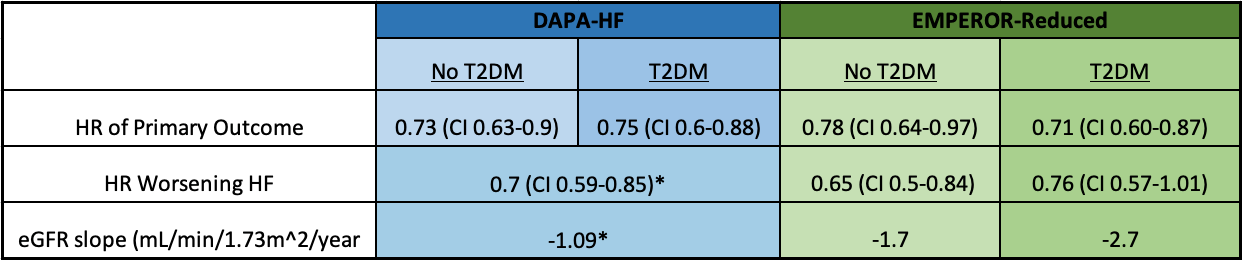
EMPEROR-Reduced was similarly designed to evaluate the cardiovascular and renal benefits of empagliflozin in patients with HFrEF, both with and without diabetes.9 The trial enrolled patients with pre-existing New York Heart Association (NYHA) class II-IV HF and ejection fraction <40%. Of the patients enrolled, 50% had T2DM, 34% had pre-diabetes with a glycosylated hemoglobin (HbA1c) of 5.7-6.4, and 15% of patients had a normal HbA1c of <5.7. Patients were generally well-optimized on guideline-directed medical therapy for HF prior to enrollment, and those with T2DM had good glucose control, with a mean HbA1c of 7.4. The study's primary endpoint was a composite of time to CV death or first HF hospitalization, with secondary endpoints that included total number of HF hospitalizations, slope of change of estimated glomerular filtration rate (eGFR), and clinically significant renal events, including need for renal replacement therapy, renal transplant, >40% decrease in eGFR, or sustained eGFR of <10-15 mL/min/1.73m2/year, depending on baseline renal function. They found that empagliflozin reduced the risk of primary outcomes in patients with and without T2DM (HR 0.75 CI 0.65-0.86), as well as risk of HF hospitalization (HR 0.70, CI 0.58-0.85) and annual rate of eGFR decline (0.55 vs. 2.28 mL/min/1.73m2/year).
In a pre-specified secondary analysis of EMPEROR-Reduced by Dr. Anker and colleagues, the lower rate of CV death or HF hospitalization, as well as adverse renal events, were found to be durable regardless of diabetes status or baseline HbA1c.10 Though the risk of cardiorenal events in the placebo arm was higher in patients with T2DM, the relative benefit of empagliflozin was similar in those with and without T2DM. Patients randomized to empagliflozin had a lower composite rate of CV death or HF hospitalization whether or not they had T2DM (HR 0.72, CI 0.60-0.87 and HR 0.78, CI 0.64-0.97, respectively). Empagliflozin similarly slowed the rate of eGFR decline in all groups significantly (by 2.2 mL/min/1.73m2 with T2DM and 1.3 mL/min/1.73m2 without T2DM) and reduced the incidence of clinical renal events irrespective of diabetes status (HR 0.53, CI 0.31-0.90 with T2DM and HR 0.42, CI 0.19-0.97 without T2DM). Similarly, HbA1c modeled as a continuous variable did not change the effect of empagliflozin on prespecified outcomes. Taken together, these data suggest that patients with HF and comorbid diabetes are at a substantially higher risk for adverse events such as CV death, hospitalization for heart failure, and worsening renal function, but that the benefits of empagliflozin extend to all patients with HF, regardless of diabetes status. Furthermore, these findings were consistent with those reported in DAPA-HF.8,11 (Table 1)
Table 1: Effect of SGLTis in DAPA-HF and EMPEROR-Reduced is consistent in those with and without T2DM
Importantly, this analysis showed that empagliflozin was not associated with hypoglycemia in either patients with or without T2DM. Consistent with prior literature, those randomized to empagliflozin had a higher risk of genitourinary infections, but there were no other disproportionate adverse effects reported in the empagliflozin arm.
Taken together with DAPA-HF, the results from this pre-specified secondary analysis of EMPEROR-Reduced strongly suggest that there is a role for SGLT2is in patients with heart failure with reduced ejection fraction, whether or not T2DM is present. Further, empagliflozin is well-tolerated in patients both with and without T2DM and does not lead to hypoglycemia in those without T2DM. In light of this information, the recently published 2021 update to the ACC's Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment urges the use of SGLT2i in patients with HFrEF regardless of diabetes status.12 Further research is necessary to identify the optimal order of medication initiation, but currently the ACC recommends starting a SGLT2i in those already optimized on other HFrEF guideline-directed medical therapy, specifically a beta blocker and angiotensin receptor-neprolysin inhibitor or angiotensin converting enzyme inhibitor. The results of recent trials compel the use of SGLT2is as HF and renal protective agents rather than simply AHAs for patients with T2DM. The initiation of a SGLT2i should be strongly considered in all patients with HFrEF, regardless of diabetes status.
References
- Gore MO, McGuire DK. Resolving drug effects from class effects among drugs for type 2 diabetes mellitus: more support for cardiovascular outcome assessments. Eur Heart J 2011;32:1832–34.
- Harrington JL, de Albuquerque Rocha N, Patel KV, Verma S, McGuire DK. Should metformin remain first-line medical therapy for patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease? An alternative approach. Curr Diab Rep 2018;18:64.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57.
- Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 2020;5:632–44.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Eng J Med 2020;383:1413-24.
- Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation 2021;143:337–49.
- Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 2021;143:298–309.
- Maddox TM, Januzzi JL, Allen LA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol 2021;77:772–810.
Clinical Topics: Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Acute Heart Failure
Keywords: Metabolic Syndrome, Diabetes Mellitus, Heart Failure, Hemoglobin A, Diabetes Mellitus, Type 2, Blood Glucose, Creatine, Hypoglycemic Agents, Prediabetic State, Pharmaceutical Preparations, Angiotensin-Converting Enzyme Inhibitors, Glomerular Filtration Rate, Natriuresis, Glucose, Kidney Transplantation, Receptors, Angiotensin, United States Food and Drug Administration, Outpatients, Stroke Volume, Hypoglycemia, Hospitalization, Protective Agents, Energy Metabolism
< Back to Listings