Paclitaxel-Coated Balloons for the Treatment of Symptomatic Central Venous Stenosis in Dialysis Patients
Central venous stenosis (CVS) is common in hemodialysis patients, often present prior to vascular access creation, usually owing to previous insertion of foreign bodies, such as central venous catheters, ports, peripherally inserted central catheters and cardiac rhythm related devices. Presence of symptoms is a prerequisite for treatment.1 Additionally, there are cases where a concomitant peripheral stenosis occurs within the vascular access circuit and after treatment, the central stenosis becomes symptomatic, a situation described as "unmasking" of CVS.2 A correlation between the type of symptomatology and the location of stenosis exists.3
The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines suggest high-pressure balloon (HPB) angioplasty as the treatment of choice for these types of lesions.4 Patency rates have been reported to be 40% at 6 months.5 In previous years, bare metal stents (BMS) implantation was suggested as a bail-out method when angioplasty failed.6 Latest guidelines, however, are averse to their use. The reason for this is the aggressive fibromuscular ingrowth known as venous neo-intimal hyperplasia (VNIH) between stent struts leading to restenosis.7 Covered stents (CS) have been used as an alternative to BMS as they restrict VNIH due to the presence of a covering barrier based on expanded polytetrafluoroethylene (ePTFE).8 The North America Society of Interventional Radiology (SIR) guidelines have evaluated patency rates for both BMS and CS and showed better results with CS placement.5 The main limitation, however, has been CS sizing for the venous system.
Paclitaxel-coated balloons (PCBs) have been used for the treatment of the dysfunctional vascular access. The results in vascular access periphery have been controversial in retrospective analyses, randomized controlled trials (RCT) and even meta-analyses.9-12 In an attempt to explain this controversy, one could argue that this is owed to the different treatment site locations extending from the inflow artery, cannulation zone, venous outflow veins and up to the central veins. Add to that, the existence of two different types of vascular access, namely the arteriovenous fistulas (AVFs) and arteriovenous grafts (AVGs); together with the different geographies, these studies make pooling of data even more challenging.13
Symptomatic CVS treatment with PCB angioplasty is gaining increasing interest. An initial study by Massmann et al. that used PCB in ten patients showed a benefit when retrospectively compared with angioplasty alone, although the study included lesions in the axillary vein which is not considered a central vein (freedom from target lesion revascularization after PCB angioplasty 12 months vs. 4 months after PBA; p=0.006).14 An RCT (20 patients in each group) from our department also showed a significant benefit of PCB angioplasty over HPB angioplasty (PCB group: 179 days vs. CBA group: 124.5 days; p=0.026).15 A retrospective single-arm study by Hongsakul et al. assessed the use of PCBs for the treatment of early recurrent stenosis (<3 months). In the 16 patients included in the analysis, primary patency was 93.8% at 6 months.16 Another interesting finding was the subgroup analysis performed in the Lutonix Global AV Registry from Karnabatidis et al. The study included 20 patients with CVS and the target lesion primary patency was 65% at 6 months.17
Lately, the results of a European, multi-center, retrospective analysis were published. The study included 86 patients from 11 centers in seven European countries.18 Median patient follow-up time was 1.0 year (IQR 0.5–2.2 years). Intervention-free period was 62.7% and access circuit survival was 87.7% at 6 months. A subgroup analysis showed that the only factor that independently influenced the primary outcome measure was the size of the balloon. More specifically, an increase of balloon diameter by 1mm improved patency by 29%. Another interesting finding of this study, with the longer follow-up available, was that mortality was 20.3% at 2 years according to Kaplan Meier survival analysis. The specific figure is below the 33% mortality rate at 2 years for the end-stage kidney disease patients in the United States. This finding is important, especially in the frame of the latest concerns regarding the use of paclitaxel devices in peripheral arterial disease showing significantly worse mortality and amputation rates.
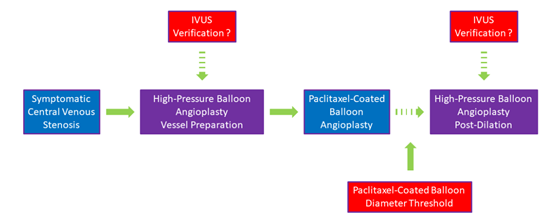
With the main characteristic of CVS being fibromuscular hyperplasia, the main limitation of PCBs remains their inability to reach HPB atmospheres. It is therefore of utmost importance that a successful vessel preparation/predilation takes place. To that end, the use of intra-vascular ultrasound (IVUS) to properly assess the vessel diameter and evaluate the result of angioplasty before using the PCB might be of value. Size availability is also another limitation with most of the companies having PCBs available up to 12mm, although post-dilation with bigger balloon diameter could occur.
To date, results of PCB use for the treatment of symptomatic CVS have been promising. For a final conclusion regarding their beneficial role in CVS treatment, data from larger RCTs based on a more systematic reporting of outcomes should be provided.
Figure 1: Courtesy of Kitrou PM.
References
- Agarwal AK. Central vein stenosis. Am J Kidney Dis 2013;61:1001-15.
- Ehrie JM, Sammarco TE, Chittams JL, Trerotola SO. Unmasking of previously asymptomatic central venous stenosis following percutaneous transluminal angioplasty of hemodialysis access. J Vasc Interv Radiol 2017;28:1409-14.
- Kundu S. Review of central venous disease in hemodialysis patients. J Vasc Interv Radiol 2010;21:963-68.
- Lok CE, Huber TS, Lee T,et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis 2020;75:S1-S164.
- Dariushnia SR, Walker TG, Silberzweig JE, et al. Quality improvement guidelines for percutaneous image-guided management of the thrombosed or dysfunctional dialysis circuit. J Vasc Interv Radiol 2016;27:1518-30.
- III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis 2001;37:S137-81.
- Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 2006;17:1112-27.
- Jones RG, Willis AP, Jones C, McCafferty IJ, Riley PL. Long-term results of stent-graft placement to treat central venous stenosis and occlusion in hemodialysis patients with arteriovenous fistulas. J Vasc Interv Radiol 2011;22:1240-45.
- Lookstein RA, Haruguchi H, Ouriel K, et al. Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med 2020;383:733-42.
- Kitrou PM, Katsanos K, Spyridonidis I, et al. Use of drug-coated balloons in dysfunctional arteriovenous dialysis access treatment: the effect of consecutive treatments on lesion patency. J Vasc Interv Radiol 2019;30:212-26.
- Trerotola SO, Lawson J, Roy-Chaudhury P, Saad TF. Drug coated balloon angioplasty in failing AV fistulas: a randomized controlled trial. Clin J Am Soc Nephrol 2018;13:1215-24.
- Fong KY, Zhao JJ, Tan E, et al. Drug coated balloons for dysfunctional haemodialysis venous access: a patient level meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg 2021;62:610-21.
- Kitrou PM, Katsanos K, Karnabatidis D. New evidence to support the use of drug coated balloons in the treatment of dysfunctional vascular access. Eur J Vasc Endovasc Surg 2021;61:540-41.
- Massmann A, Fries P, Obst-Gleditsch K, Minko P, Shayesteh-Kheslat R, Buecker A. Paclitaxel-coated balloon angioplasty for symptomatic central vein restenosis in patients with hemodialysis fistulas. J Endovasc Ther 2015;22:74-79.
- Kitrou PM, Papadimatos P, Spiliopoulos S, et al. Paclitaxel-coated balloons for the treatment of symptomatic central venous stenosis in dialysis access: results from a randomized controlled trial. J Vasc Interv Radiol 2017;28:811-17.
- Hongsakul K, Akkakrisee S, Bannangkoon K, Boonsrirat U, Premprabha D, Juntarapatin P. Results of drug-eluting stent in significant restenosis of the hemodialysis access: an initial study. Semin Dial 2021;Jun 16:[Epub ahead of print].
- Karnabatidis D, Kitrou PM, Ponce P, et al. A multicenter global registry of paclitaxel drug-coated balloon in dysfunctional arteriovenous fistulae and grafts: 6-month results. J Vasc Interv Radiol 2021;32:360-68.
- Kitrou PM, Steinke T, El Hage R, et al. Paclitaxel-coated balloons for the treatment of symptomatic central venous stenosis in vascular access: results from a European, multicenter, single-arm retrospective analysis. J Endovasc Ther 2021;28:442-51.
Clinical Topics: Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Interventions and Vascular Medicine
Keywords: Retrospective Studies, Polychlorinated Biphenyls, Constriction, Pathologic, Central Venous Catheters, Axillary Vein, Peripheral Arterial Disease, Paclitaxel, Polytetrafluoroethylene, Dilatation, Radiology, Interventional, Follow-Up Studies, Hyperplasia, Veins, Angioplasty, Balloon, Renal Dialysis, Kidney Failure, Chronic, Stents, Arteries, Kidney, Arteriovenous Fistula, Registries, Registries, Foreign Bodies, Amputation, Survival Analysis, Outcome Assessment, Health Care
< Back to Listings