A Novel Multimodality Imaging Approach For Detecting Remodeling Changes in HCM: Insights from the VANISH Trial
Quick Takes
- Hypertrophic cardiomyopathy is a disorder in which abnormal cardiac muscle architecture results in progressive asymmetric left ventricular hypertrophy, diastolic dysfunction, and myocardial injury.
- Valsartan appears to be a safe and effective treatment in preventing adverse remodeling in patients with early stage, sarcomeric hypertrophic cardiomyopathy.
- The incorporation of a multimodality cardiac imaging and biomarker composite z-scores can aid in the detection of treatment-related changes in cardiac remodeling in clinical trials.
Background
Hypertrophic cardiomyopathy (HCM) is a disorder in which derangements in cardiomyocyte architecture result in asymmetric left ventricular hypertrophy, myocardial fibrosis, and ultimately heart failure and sudden cardiac death. This disorder, which is most commonly caused by mutations encoding cardiac sarcomere proteins, is one of the most common genetic cardiovascular disorders, affecting up to 1 in 500 individuals.1
Despite its frequent occurrence in the general population, current treatments for HCM are largely supportive aside from the use of implantable cardiac defibrillators which have been shown to reduce the incidence of sudden cardiac death in this population. Current pharmacologic treatments in HCM are mainly aimed at reducing symptoms related to left ventricular outflow tract obstruction. There is little evidence that these therapies can alter the disease trajectory or prevent adverse cardiac remodeling. One limitation of previous studies has been their reliance on singular disease markers such as left ventricular mass or wall thickness. As HCM often progresses slowly in adulthood, it may be difficult to detect significant changes in single parameters, particularly in short-term follow up.2 Another limitation has been the predominant inclusion of patients with late-stage disease in prior studies. Earlier treatment, prior to the onset of irreversible chamber remodeling, may potentially lead to better therapeutic response.
Valsartan and Cardiac Remodeling in HCM
Angiotensin receptor inhibitors (ACE) and receptor blockers (ARB) have been proposed as a potential treatment for earlier stage HCM. ACE/ARB have been shown to inhibit (TGF-B), a pro-fibrotic cytokine which has been implicated in the development of myocyte hypertrophy and fibrosis in HCM in both humans and mouse models.3,4 Previous studies, mainly involving later stage patients, have not been able to show benefit with ACE/ARB in HCM.5,6 However, it is unknown whether these therapies can affectively slow adverse remodeling in earlier stage disease.
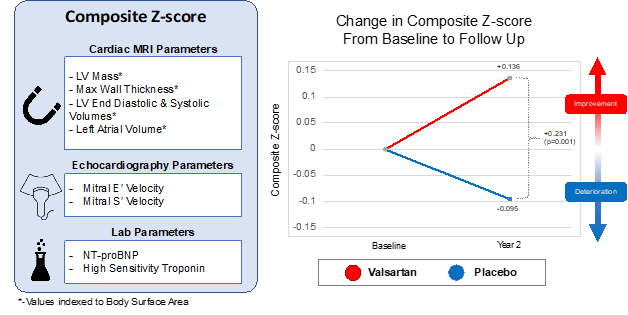
In the recent edition of Nature Medicine, Carolyn Ho et al. assessed the impact of valsartan on left ventricular chamber remodeling in patients with early stage, sarcomeric HCM.7 The authors performed a multi-site, randomized control trial of valsartan versus placebo in 178 patients with sarcomeric HCM. The primary outcome, a composite age-adjusted multi-modality cardiac imaging and serum biomarker z-score system composed of indexed left ventricular mass, left atrial volume, left ventricular end diastolic and end systolic volume, maximal wall thickness, mitral annular E'/S', N-terminal pro BNP (NT-proBNP), and troponin, was compared between patients receiving valsartan and controls (Figure 1). Each parameter was measured on the cardiac imaging modality that is best suited to make the measurement (i.e., the masses and volumes were measured using cardiac magnetic resonance and the diastolic parameters were measured using echocardiography).
Figure 1
To enroll earlier stage patients, those older than age 30 years or whom had a maximal wall thickness >20 mm were excluded. However, during the enrollment period, they increased the maximal age to 45 years and wall thickness to <25 mm to aid in recruitment.
At 2 years follow up, they found significantly better multi-modality cardiac imaging and serum biomarker composite z-scores in valsartan-treated patients as compared to controls (Figure 1). In univariate analysis, NT-proBNP, mitral annular E', and left ventricular end diastolic volume7 was significantly different between treatment groups. In sensitivity analysis, the treatment effect was strongest in younger patients and in those with lower maximal wall thickness. Importantly, there were no significant differences in adverse side effects between the two groups.
Discussion:
The authors conclude that treatment with valsartan is associated with attenuated adverse left ventricular remodeling in patients with early stage sarcomeric HCM. This treatment effect was observed across all genders and age groups in pre-specified subgroup analysis. The authors hypothesize that their selection of earlier stage patients may explain why their trial showed benefit despite previous negative studies.
Ho et al. should be congratulated on their novel approach to this trial. In contrast to previous studies which have largely relied on singular endpoints, the authors used a composite z-score comprised of multimodality cardiac imaging and serum biomarkers which are known to be deranged in HCM (Figure 1). This allowed them to assess the overall impact of valsartan on global cardiac remodeling and injury. By evaluating multiple parameters, a composite score can amplify the measured treatment effect and allow for improved statistical power to detect inter-group differences. This is particularly valuable in rarer disorders such as sarcomeric HCM in which the assembly of large, randomized control trials is less feasible. Lastly, the use of a multiparametric composite score may help to identify changes in less clinically obvious variables which might otherwise not be discovered with univariate endpoints.
As the composite z-score is affected by all parameters equally, one must be careful in selecting parameters, prioritizing the inclusion of only the most clinically important variables in the z-score. The inclusion of less important parameters may lead to the detection of changes which, while significant, are not clinically important. The authors address this with a pre-specified univariate analysis in which they found that of the individual parameters assessed, only NT-proBNP, left ventricular end diastolic volume, and mitral E' velocity7 differed significantly between treatment and control groups.
In conclusion, valsartan is associated with a reduction in adverse left ventricular remodeling in patients with early sarcomeric HCM. Valsartan appears to be a safe and effective treatment to prevent disease progression in this patient population.
References
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65:1249-54.
- Ho CY, Cirino AL, Lakdawala NK, et al. Evolution of hypertrophic cardiomyopathy in sarcomere mutation carriers. Heart 2016;102:1805-12.
- López B, González A, Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation 2010;121:1645-54.
- Teekakirikul P, Eminaga S, Toka O, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest 2010;120:3520-29.
- Axelsson A, Iversen K, Vejlstrup N, et al. Efficacy and safety of the angiotensin II receptor blocker losartan for hypertrophic cardiomyopathy: the INHERIT randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2015;3:123-31.
- Shimada YJ, Passeri JJ, Baggish AL, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail 2013;1:480-87.
- Ho CY, Day SM, Axelsson A, et al. Valsartan in early-stage hypertrophic cardiomyopathy: a randomized phase 2 trial. Nat Med 2021;27:1818-24.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Implantable Devices, SCD/Ventricular Arrhythmias, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Echocardiography/Ultrasound
Keywords: Ventricular Remodeling, Angiotensin Receptor Antagonists, Hypertrophy, Left Ventricular, Sarcomeres, Follow-Up Studies, Control Groups, Myocytes, Cardiac, Stroke Volume, Angiotensin-Converting Enzyme Inhibitors, Cardiomyopathy, Hypertrophic, Echocardiography, Heart Failure, Disease Progression, Receptors, Angiotensin, Magnetic Resonance Spectroscopy, Fibrosis, Heart Atria, Defibrillators, Biomarkers, Cytokines, Troponin, Valsartan
< Back to Listings