Non-Alcoholic Fatty Liver Disease: An Emerging Cardiovascular Risk Enhancer
Quick Takes
- Understand the intersection between non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease.
- Learn the diagnostic and management options for NAFLD that will impact cardiovascular outcomes.
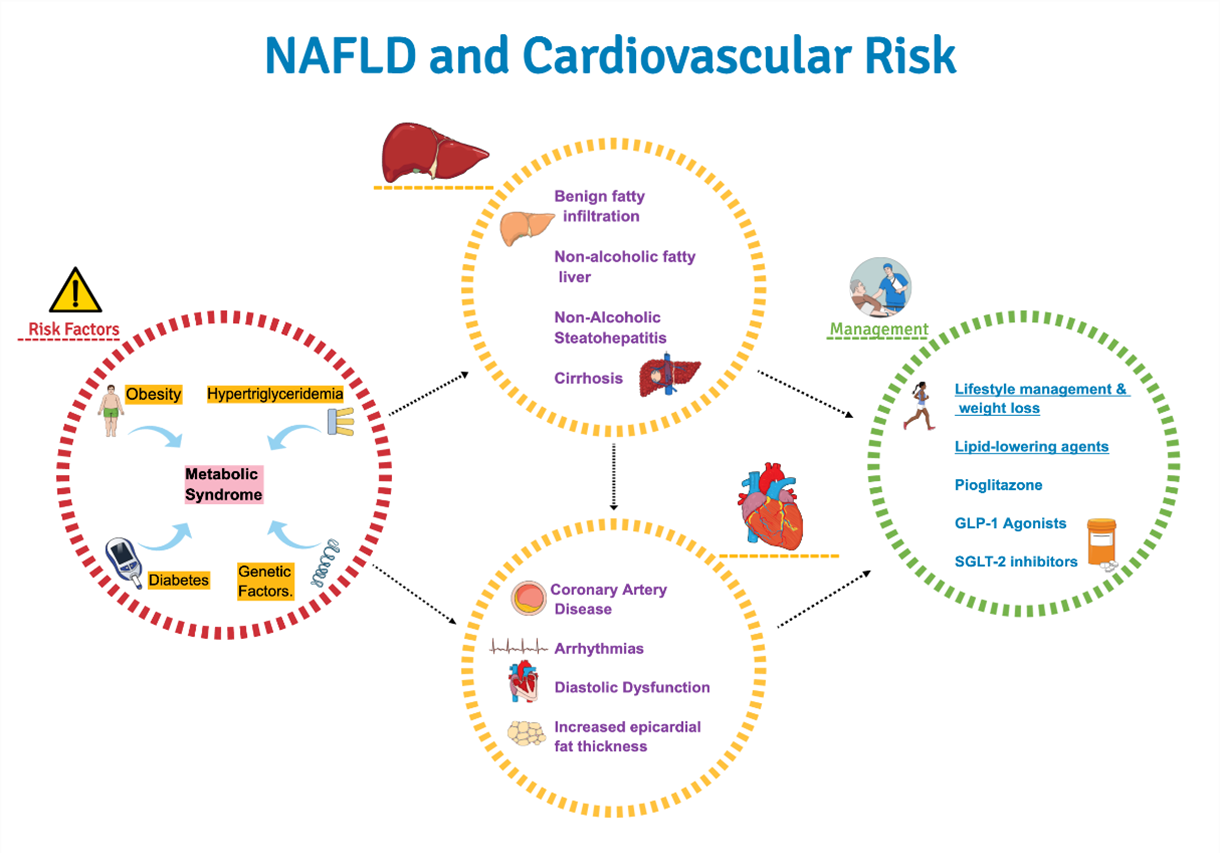
The recent American Heart Association Statement on non-alcoholic fatty liver disease (NAFLD) and cardiovascular risk aims to raise awareness that NAFLD should be considered as a cardiovascular risk marker and risk enhancer.1 NAFLD refers to a continuum of hepatic conditions characterized by fatty infiltration of hepatocytes (hepatic steatosis) in the absence of secondary causes of hepatic fat accumulation such as alcohol use or steatogenic medication use.2,3 NAFLD ranges from non-alcoholic fatty liver disease which is a benign fatty infiltration of hepatocytes (>5% of fatty infiltration of hepatocytes), to non-alcoholic steatohepatitis (NASH) which is characterized by hepatic steatosis with accompanying cellular ballooning and lobular inflammation with or without fibrosis. NASH may further progress to cause fibrosis and ultimately, cirrhosis.4
Epidemiology and Natural History
Worldwide, the estimated prevalence of NAFLD is around 25%, with South Asia having the highest numbers (33%). In the United States (US), the estimated prevalence of NAFLD and NASH is 30% and 5% respectively.5 Racial and ethnic differences exist, with Hispanics, especially those of Mexican origin, having the highest prevalence (33%) followed by Caucasians (12.5%) and African-Americans (11.6%). NAFLD is the most common etiology for chronic liver disease and the second most common indication for liver transplants in the US6 and its prevalence is expected to increase with increasing rates of obesity and rising prevalence of type-2 diabetes mellitus (T2DM).7
Cardiovascular disease (CVD) is the most common cause of death in NAFLD despite high rates of liver-related and overall mortality. There is a bidirectional relationship between cardiovascular risk factors of obesity, insulin resistance, T2DM and NAFLD, whereby higher degree of metabolic risk portends increased severity of liver disease.8 Around 25% of patients with NAFLD progress to NASH and fibrosis and 7% progress to cirrhosis and end-stage liver disease. The degree and extent of fibrosis influences severity of long-term outcomes including atherosclerotic cardiovascular disease (ASCVD) outcomes.
Intersection of NAFLD and CVD
NAFLD is categorized as a risk enhancer rather than risk factor since the risk-modulating effect has not been quantified numerically, despite demonstration that it has an incremental adverse impact on ASCVD risk over and above traditional risk factors. Moreover, studies showing decrease in ASCVD with treatment of NAFLD are unavailable at this time which are integral to classifying this as risk factor. Pathophysiology of NAFLD begins with genetic predilection with superimposed lifestyle factors. High dietary consumption of saturated fat and fructose in the setting of insulin resistance promotes an increase in free fatty acid (FFA) traffic to the liver which triggers hepatic lipotoxicity, paralleling fat accumulation in other tissues such as epicardium, pancreas, and skeletal muscle. Intrahepatic accumulation of FFA leads to mitochondrial dysfunction, production of reactive oxygen species, and activation of the renin angiotensin aldosterone system (RAAS), which promotes clinical syndromes of atrial fibrillation (AF), diastolic heart failure and ASCVD.9 In fact, recurrence of AF, subclinical atherosclerosis and high-risk plaque features are higher in NAFLD population compared to controls.10 NASH and its severity is also related to chronic kidney disease (CKD). The relationship of NAFLD with CVD and CKD is independent of T2DM and obesity.11
Diagnosis of NAFLD
Many patients with NAFLD have normal plasma AST and ALT levels and remain undiagnosed, but the NAFLD fibrosis score and fibrosis-4 score, estimated from common blood tests, can predict fibrosis with a high degree of accuracy.12 Hepatic ultrasonography has a sensitivity of 79.7% and vibration-controlled transient elastography (FibroScan) can detect liver stiffness, an index of advanced fibrosis, with an accuracy of 79%.13 If there is a high degree of suspicion based on risk factors and transaminase levels, ultrasound imaging and/or FibroScan may be indicated.14
Management
- Non-Pharmacological Interventions
The cornerstone of prevention and treatment of NAFLD and NASH remains weight loss via lifestyle modifications. Histological improvement in NAFLD is proportional to amount of weight loss and a >7-10% weight reduction significantly impacts liver steatosis, inflammation and necrosis.15 Adherence to a Mediterranean diet is inversely associated with steatosis and fibrosis and decreases 10-year risk of T2DM and CVD in NAFLD subjects.16 Fructose intake should be minimized even though it has a low glycemic index, because it aggravates weight gain, stimulates intrahepatic triglyceride (TG) accumulation, and has been associated with worsening fibrosis and progression to NASH. Restriction of dietary fat intake can lower TGs and especially if not replaced with excess simple carbohydrate intake. Weight-loss medications and bariatric surgery may have a positive impact on NAFLD but have not been well studied. - Pharmacological Interventions
Anti-Diabetic Agents
Glucagon-like Peptide-1 receptor agonists (GLP1-RA) are recommended in patients with NASH, obesity and/or T2D given their ability to promote weight loss, enhance hepatic insulin sensitivity and reduce TG accumulation in the liver. Liraglutide and semaglutide have significant biopsy-proven histological benefit along with weight loss and glycemic control in diabetics although reduction in fibrosis has not been proven.17,18 Animal studies have shown reduction in steatohepatitis with sodium-glucose co-transporter 2 (SGLT-2) inhibitors but there is no human data to support its use in NAFLD. Pioglitazone is safe and effective in diabetics with NASH and improves insulin sensitivity in adipose tissue, liver, and muscle, despite some weight gain. Among all anti-diabetic therapies, pioglitazone showed the greatest decrease in TG content in liver and improvement in histology followed by GLP1-RA.19 Although metformin improves insulin sensitivity, there is no effect on hepatic fat content or fibrosis.
Lipid-Lowering Agents
Since the initial concerns with use of statins in the presence of liver pathology, there have been a number of prospective studies with atorvastatin,20 all notable for significant improvement in transaminase as well as cholesterol levels with a decrease in hepatic fatty infiltration in subgroup, post-hoc analysis. Use of statins in those with mildly elevated ALT at baseline is associated with better outcomes and overall, the cardiovascular and hepatic benefits of statin use far outweighs the minimal risk of liver failure. In patients with high ASCVD risk or known CVD, statins are safe to use in those with chronic liver disease, with close monitoring of liver enzymes. Other lipid-lowering therapies such as ezetimibe, fibrates, bempedoic acid and PCSK-9 inhibitors have not been studied in NAFLD.
Antioxidants and Investigational Agents
Vitamin E improves liver histology in nondiabetic adults with biopsy-proven NASH but is not recommended for ASCVD risk reduction. Other investigational agents include elafibranor and cenicriviroc which enhance insulin sensitivity, reduce hepatic inflammation, and fibrosis.
Screening
Clinical practice guidelines do not recommend screening for NAFLD in the general population, but it may be reasonable in high-risk groups such as T2D with metabolic syndrome traits and first-degree relatives with NASH cirrhosis.
Summary
Presence of NAFLD increases CVD risk and appropriate management is indicated to improve liver and CV outcomes. In patients with NAFLD, studies focused on improvement in hepatic histology are warranted with all classes of lipid lowering therapies and antidiabetic drugs. Since NAFLD can be considered a risk enhancer, its presence should dictate more aggressive lifestyle interventions and a lower threshold for medical management of lipids, diabetes, and obesity.
References
- Duell PB, Welty FK, Miller M, et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2022;42:e168-e185.
- Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology 2016;150:1769-77.
- Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord 2022;22:63.
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330-44.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-57.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 2020;158:1851-64.
- Pais R, Maurel T. Natural history of NAFLD. J Clin Med 2021;10:1161.
- Shroff H, VanWagner LB. Cardiovascular disease in nonalcoholic steatohepatitis: screening and management. Curr Hepatol Rep 2020;19:315-26.
- Donnellan E, Cotter TG, Wazni OM, et al. Impact of Nonalcoholic fatty liver disease on arrhythmia recurrence following atrial fibrillation ablation. JACC Clin Electrophysiol 2020;6:1278-87.
- Papademetriou M, Athyros VG, Geladari E, Doumas M, Tsioufis C, Papademetriou V. The co-existence of NASH and chronic kidney disease boosts cardiovascular risk: are there any common therapeutic options? Curr Vasc Pharmacol 2018;16:254-68.
- Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a Review. JAMA 2020;323:1175-83.
- Shannon A, Alkhouri N, Carter-Kent C, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr 2011;53:190-95.
- Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717-30.
- Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity 2009;17:2162-68.
- George ES, Forsyth A, Itsiopoulos C, et al. Practical dietary recommendations for the prevention and management of nonalcoholic fatty liver disease in adults. Adv Nutr 2018;9:30-40.
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-90.
- Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113-24.
- Kim KS, Lee BW. Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease. Clin Mol Hepatol 2020;26:430-43.
- Pedersen TR, Faergeman O, Kastelein JJP, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: a randomized controlled trial. JAMA 2005;294:2437-45.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Prevention, Atrial Fibrillation/Supraventricular Arrhythmias, Hypertriglyceridemia, Lipid Metabolism, Nonstatins, Novel Agents, Statins, Acute Heart Failure, Chronic Heart Failure, Echocardiography/Ultrasound, Diet
Keywords: Non-alcoholic Fatty Liver Disease, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Prospective Studies, Antioxidants, Atorvastatin, Cardiovascular Diseases, Fatty Acids, Nonesterified, Glucagon-Like Peptide-1 Receptor, Insulin Resistance, Liraglutide, Pioglitazone, Reactive Oxygen Species, Renin-Angiotensin System, Sodium-Glucose Transporter 2 Inhibitors, Elasticity Imaging Techniques, Metabolic Syndrome, Prevalence, American Heart Association, Atrial Fibrillation, Cause of Death, Diet, Mediterranean, End Stage Liver Disease, Glycemic Control, Glycemic Index, Heart Failure, Diastolic, Liver Transplantation, Risk Factors, Liver Cirrhosis, Hepatocytes, Adipose Tissue, Obesity, Diabetes Mellitus, Type 2, Heart Disease Risk Factors, Mitochondria, Renal Insufficiency, Chronic, Life Style, Weight Loss, Atherosclerosis, Risk Reduction Behavior, Carbohydrates, Transaminases, Triglycerides, Dietary Fats, Weight Gain, Hematologic Tests, Cholesterol, Pericardium, Ezetimibe, Vitamin E, Metformin, Fibric Acids, Glucose
< Back to Listings