Pre-Procedural Interventional TEE: Tricuspid Valve
Quick Takes
- Transesophageal echocardiography allows identification of tricuspid regurgitation mechanism, severity and anatomy, and determination of suitability for transcatheter edge-to-edge repair versus transcatheter valve replacement.
- Two- and three-dimensional transesophageal echocardiography have led to refinements in tricuspid valve leaflet nomenclature and a greater appreciation of variations in tricuspid valve anatomy such as clefts/folds that affect percutaneous interventions.
- Transesophageal echocardiography imaging of the tricuspid valve can be challenging due to the anterior and inferior location of the valve and may indicate the need for combined transesophageal and intracardiac echocardiography for procedural guidance.
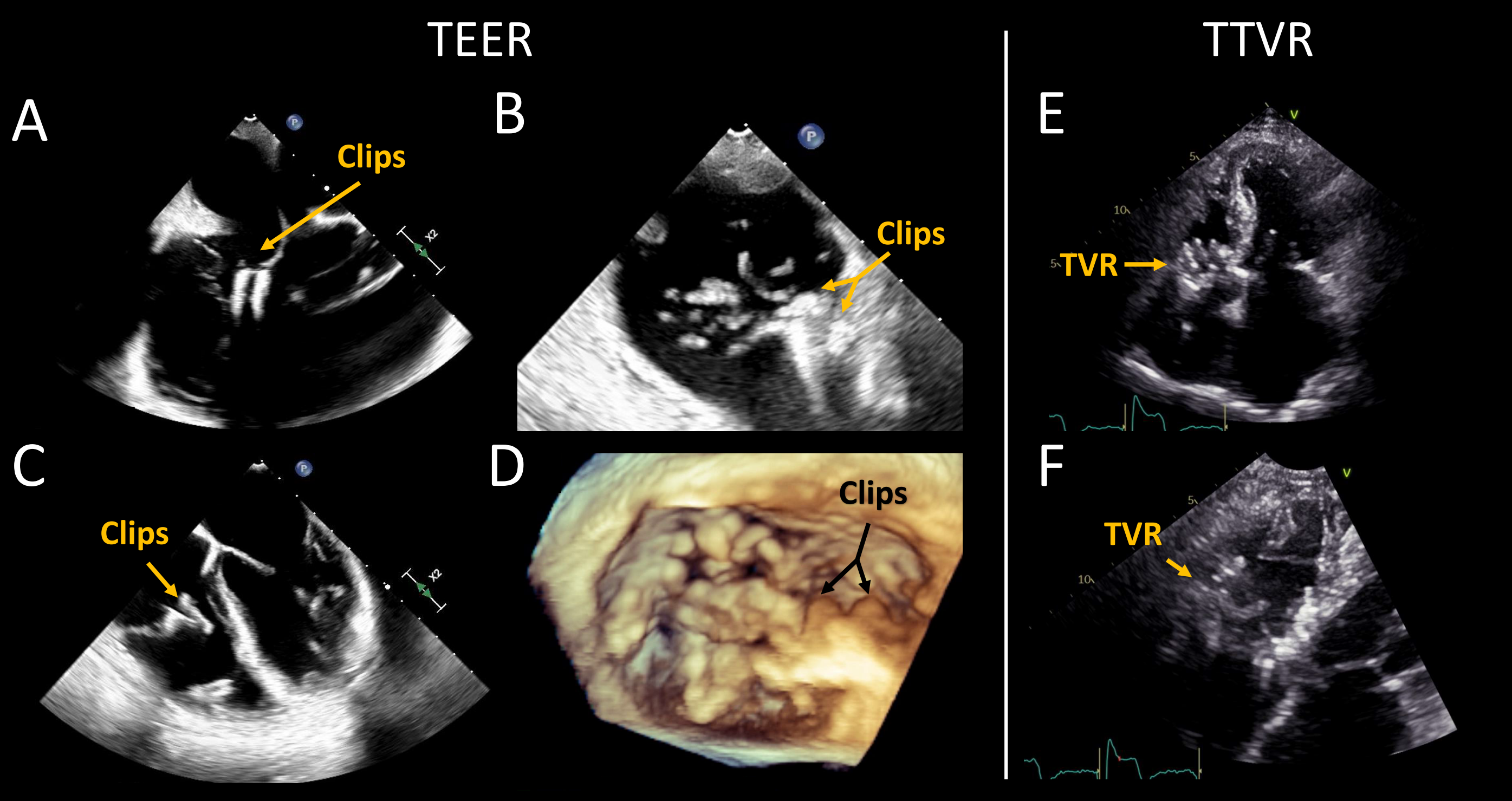
Transcatheter treatment of tricuspid valve (TV) regurgitation is gaining in popularity as an alternative for patients unable to undergo surgery. Currently, transcatheter edge-to-edge repair (TEER, Figure 1A-B-C) is the most commonly performed, while other devices such as transcatheter TV replacement (TTVR, Figure 1D), restrictive annuloplasty, and heterotopic valve implantation (caval devices) are still under investigation.1 Transesophageal echocardiography (TEE) plays a pivotal role in the pre-procedural evaluation. This article will review the role of TEE in the pre-procedure evaluation of the TV for these interventions.
Figure 1
(A) Mid-esophageal 4-chamber, (B) mid-esophageal commissural, and (C) transgastric short-axis two-dimensional echocardiographic images of the tricuspid valve (TV) in a patient with a previous edge-to-edge repair (TEER) (2 clips, arrows). (D) Mid-esophageal three-dimensional echocardiographic image of a TV post TEER. (E-F) Transthoracic apical 4-chamber two-dimensional echocardiographic image in a patient with a previous transcatheter tricuspid valve replacement (TTVR). Acronyms: TEER: transcatheter edge-to-edge repair, TR: tricuspid regurgitation, TTVR: transcatheter tricuspid valve replacement, and TV: tricuspid valve. Courtesy of Belzile D, Henry M, Lang R, Tsang W.
The TV is a complex dynamic structure comprised of leaflets, annulus, subvalvular apparatus, the right atrium, and the right ventricle.2-4 Two- (2D) and three-dimensional (3D) TEE are the primary imaging modalities used to assess the TV for percutaneous procedures. Guidelines for TV assessment for percutaneous procedures were published in 2022.4 The aims of the pre-procedural TEE are to:
- Quantify TR severity and mechanism
- Determine the TV leaflet morphology and possible anatomic variations
- Assess annular and adjacent anatomy
- Exclude any potential adverse features
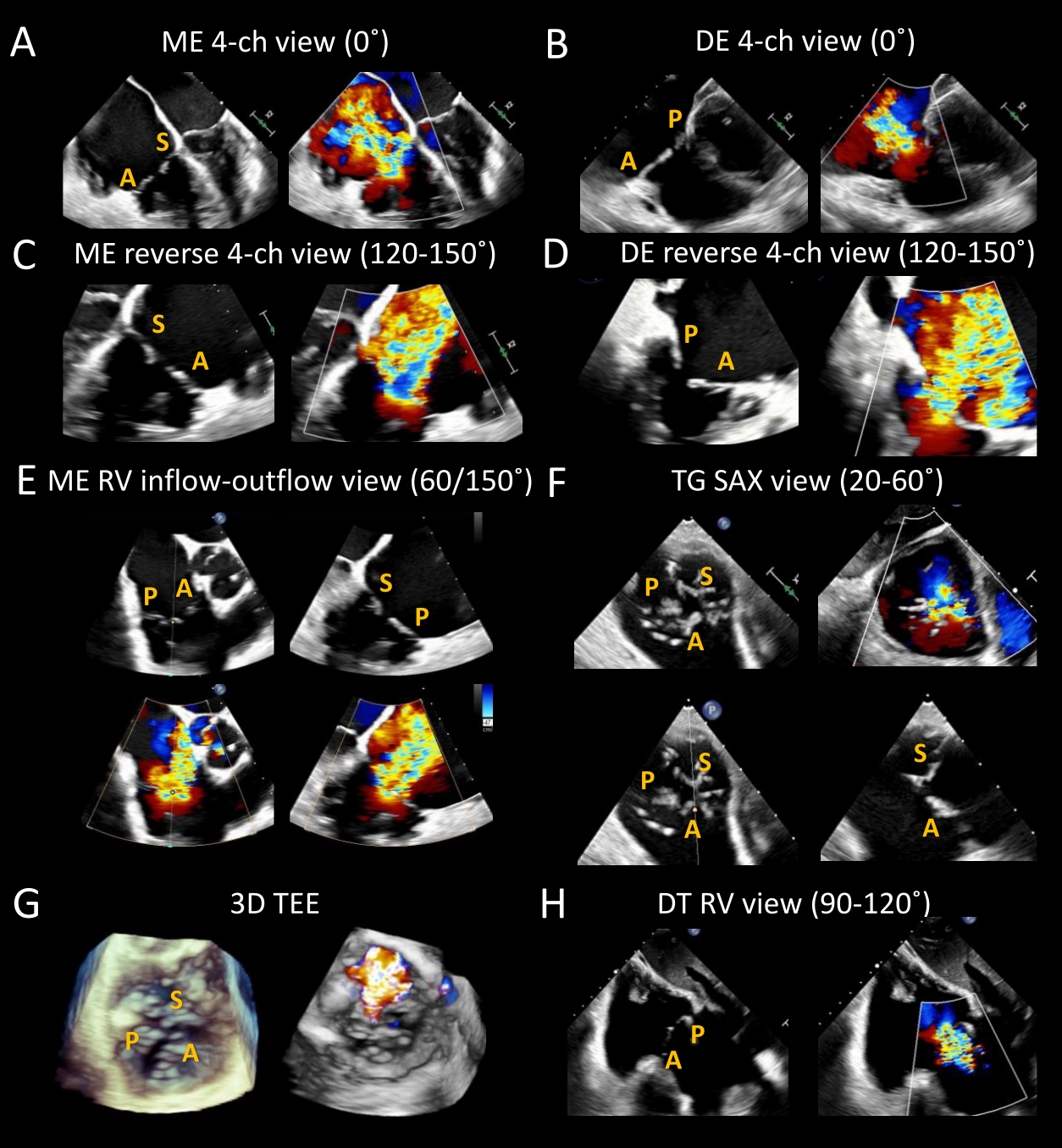
Systematic acquisition of standard views is recommended to allow comprehensive TV assessment and is essential to procedure/device selection. Four levels of TEE imaging are generally obtained: mid-esophageal (ME), deep esophageal (DE), transgastric (TG), and deep transgastric (DT).4,5 Both 2D and color Doppler images should be acquired at each level to delineate the TR mechanism and severity. The main required views are the ME 4-chamber view (0°), the ME commissural view (50-70°), the DE 4-chamber view (0°), and the TG short-axis view (0-30°) (Figure 2).
Figure 2
Standard transesophageal and transgastric views for tricuspid valve (TV) preprocedural assessment. (A) The mid-esophageal (ME) TV 4-chamber view is obtained at 0° and usually images the septal and anterior leaflets. (B) ME reverse 4-chamber view can be obtained by increasing the probe angle to 120-150°. This view, also known as the dedicated grasping view, may be useful to identify a target for intervention. (C) The ME right ventricular inflow-outflow view (TV commissural view), achieved by changing the angle to 50-70°, allows a comprehensive assessment of all three tricuspid leaflets. A biplane image adjacent to the aorta will image the anterior and septal leaflets in the orthogonal plane, and a biplane image adjacent to the posterolateral wall will instead image the posterior and septal leaflets. The septal leaflet length can be measured, and a possible target for intervention can be identified while visualizing eventual obstacles to intervention (chords, clefts/folds, calcifications, artifacts). (D) Three-dimensional (3D) TV images can be obtained from any view where the TV is well visualized. 3D images may prove useful to assess the TV morphology, tricuspid regurgitation severity, and identify any adverse features such as clefts/folds. (E and F) Deep esophageal 4-chamber and reverse 4-chamber views can also be obtained by carefully inserting the probe into the distal esophagus. These views usually allow an improved Doppler assessment of the regurgitant jet because the interrogation beam is typically better aligned with the tricuspid regurgitation jet. (G) Different imaging planes are usually obtained from the transgastric window: RV inflow-outflow view (0°), single-plane short-axis view (20-60°), and right ventricle view (90-120°). The short-axis view allows a visual assessment of the number of leaflets, their morphology, and the regurgitation jet mechanism. If pacemaker wires are present, their exact location and possible leaflet impingement can be visualized. The coaptation gap is usually well seen and can be accurately measured. Furthermore, this view helps identify the targets for transcatheter edge-to-edge repair and any potential obstacles such as prominent chords. (H) Finally, deep transgastric view of the TV can be acquired by progressing the probe deeper into the stomach with rightward anterior flexion. This view is frequently used to assess TV function using Doppler parameters and can also be utilized to acquire 3D TV images. Acronyms: 3D: three-dimensional, 4-ch: four-chamber, A: anterior leaflet, DE: deep-esophageal, DT: deep-transgastric, ME: mid-esophageal, P: posterior leaflet, RV: right ventricle, SAX: short-axis, S: septal leaflet, TEE: transesophageal echocardiogram, TG: transgastric, and TV: tricuspid valve. Courtesy of Belzile D, Henry M, Lang R, Tsang W.
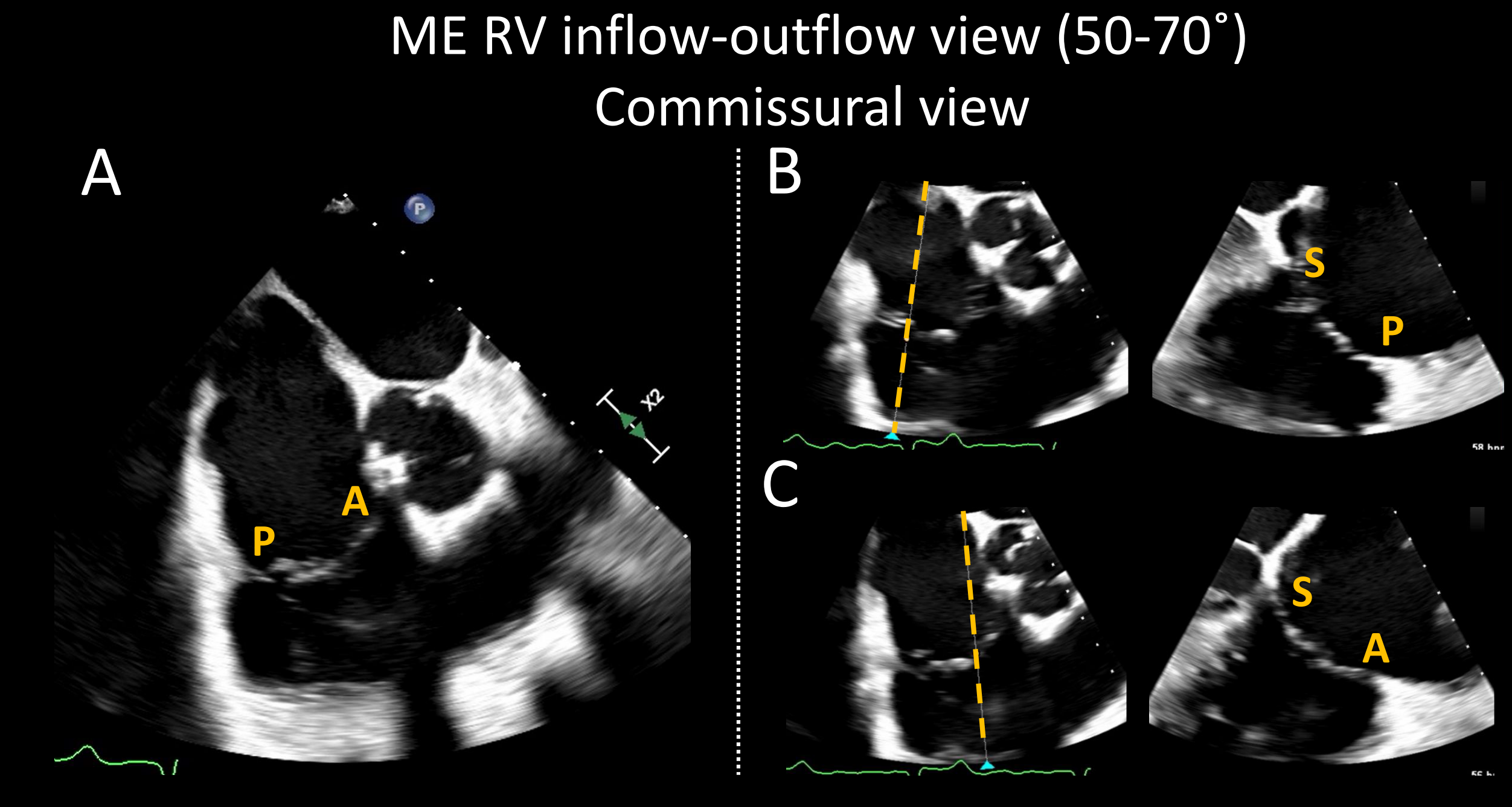
3D multiplane mode should be used on the ME commissural views at the site of the TR jet to visualize the orthogonal 4-chamber view, which allows jet localization between the anterior-septal or the posterior-septal leaflets (Figure 3). This orthogonal 4-chamber view provides the leaflet grasping view, which is needed to determine TEER device placement and feasibility. It is on this view that the TR mechanism, the leaflet gap size, septal leaflet length, and the degree of septal leaflet curling can be established. The presence/absence of significant calcifications or prominent chords can also be confirmed. The TG short axis view with color allows localization of the jet, and without color, identification of clefts and chords that may limit procedural success. During the procedure, this view is used to orient the TEER device arms perpendicular to the coaptation line.
Figure 3
(A) Mid-esophageal right ventricular inflow-outflow view, also known as the commissural view, can be obtained by forward mechanical rotation between 50-70°. Systematic biplane evaluation of the tricuspid valve on this view (B and C) allows an accurate assessment of all three leaflets and potential adverse features such as curled leaflet, calcification, and prominent chord can be identified. Acronyms: A: anterior leaflet, ME: mid-esophageal, P: posterior leaflet, RV: right ventricle, and S: septal leaflet. Courtesy of Belzile D, Henry M, Lang R, Tsang W.
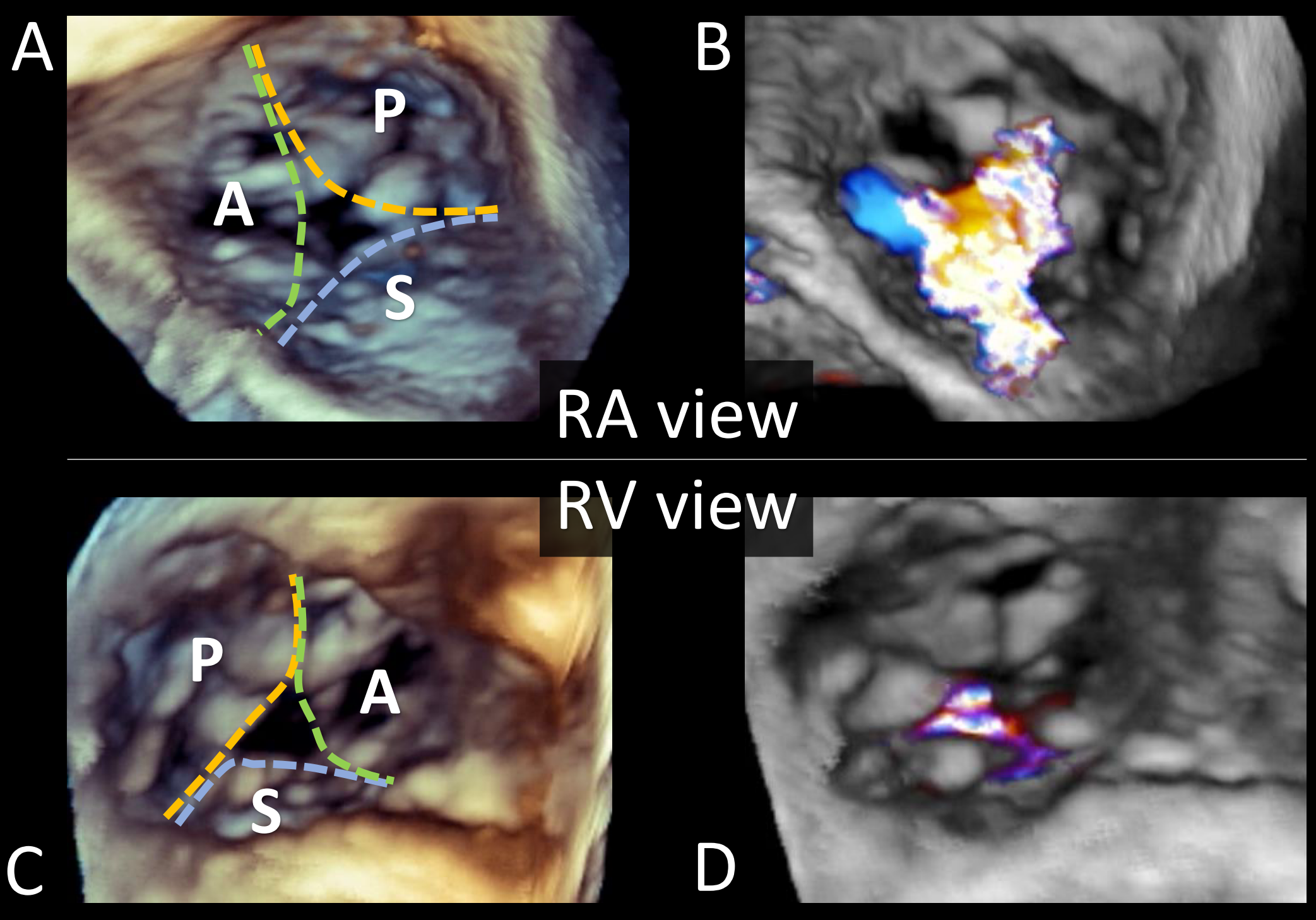
3D TEE imaging has improved our understanding of TV anatomy and TR severity. 3D TV datasets should be acquired from the TEE window that allows the best visualization of the leaflets throughout the cardiac cycle in both orthogonal views. En face visualization of the TV facilitates leaflet identification, avoiding the need to rely on adjacent structures or mentally reconstruct the valve from 2D imaging planes (Figure 4).
Figure 4
(A-B) Transesophageal short-axis three-dimensional (3D) echocardiographic image of the tricuspid valve (TV) as view from the right atrium and (C-D) the right ventricle, with and without color Doppler. 3D images can be attained from every imaging window (mid-esophageal, deep-esophageal, transgastric, and deep-transgastric), if the two-dimensional resolution is adequate. The 3D en face view of the TV can be oriented with the interatrial septum placed inferiorly at 6 o'clock, regardless of the atrial or ventricular orientation or from the atrial perspective with the aorta at the 5 o'clock position. 3D images generally allow visualization of all TV leaflet and their morphology. Different measurements can also be obtained from 3D data sets (3D planimetry and 3D vena contracta area). Acronyms: 3D: three-dimensional, A: anterior leaflet, P: posterior leaflet, RA: right atrium, RV: right ventricle, S: septal leaflet, and TV: tricuspid valve. Courtesy of Belzile D, Henry M, Lang R, Tsang W.
It also allows recognition of TV leaflet clefts, indentations, and deep folds. Studies have found that the TV has three well-defined leaflets in only 54% of cases.3 Two posterior, septal, or anterior leaflets are found in 32%, 4%, and 3% of patients, respectively.3 Bileaflet TVs are rare, representing only 5% of cases.3 Typically, the septal leaflet is attached to the interventricular septum, and the anterior papillary muscle delimits the separation between the anterior and posterior leaflets. Furthermore, each leaflet can be subdivided, with numbering starting at the anteroseptal commissure for the anterior (A1/A2) and septal (S1/S2) leaflets, and the anterior papillary muscle for the posterior leaflet (P1/P2).
3D images can elucidate the role of pacemaker leads in causing TR and whether their location will limit intervention (Video 1).6 Also, classification of TR severity is improved using 3D quantification of anatomic and effective regurgitant orifice areas.2,7-10 Finally, 3D visualization of the TV could reduce the intraprocedural need for TG images as TEER device orientation can be determined from the 3D en face renderings or multiplane reconstructions.
Video 1
(A-B) Transesophageal short-axis three-dimensional (3D) echocardiographic image of the tricuspid valve (TV) as view from the right atrium and (C-D) the right ventricle, with and without color Doppler. A pacemaker lead is visualized interfering with the anterior leaflet mobility and coaptation, resulting in severe functional tricuspid regurgitation. Acronyms: 3D: three-dimensional, A: anterior leaflet, P: posterior leaflet, RA: right atrium, RV: right ventricle, S: septal leaflet, and TV: tricuspid valve. Courtesy of Belzile D, Henry M, Lang R, Tsang W.
An ideal candidate for TEER has good TEE imaging windows, primary TR with leaflet prolapse or secondary TR with normal leaflets. Ideally, the coaptation gap is under 4 mm (maximum 7 mm), the coaptation depth <10 mm, and leaflets >10 mm. The main TR jet should be central or between the anterior-septal leaflets.11 Candidate for TTVR are usually patients considered unsuitable for optimal TEER (i.e., coaptation gaps >10 mm, severe leaflet tethering, or pacemaker-induced TR) with anatomy amenable to TTVR (i.e., tricuspid annulus diameter between 36-52 mm [with valve oversizing <10%], right atrium length ≥6 or 7 cm for transatrial or transjugular access, right internal jugular vein to superior vena cava distance ≥14 mm, without any significant risk of right coronary artery compromise or right ventricular outflow tract obstruction).1,12
Despite the strengths of TEE imaging, there are technical and anatomic limitations. TEE assessment can be challenging due to the anterior position of the TV, making it prone to technical limitations (i.e., far-field imaging, lateral resolution, acoustic shadowing, etc.).8 Also, prior cardiac interventions or structural abnormalities, such as aortic or mitral valve replacement, lipomatous interatrial septum, or significant mitral-aortic calcifications, can lead to artifacts that limit TV visualization.8 Finally, some TV procedures also require assessment of extracardiac vascular structures, such as the vena cava, which is limited with TEE only.8
Complementary imaging that can address TV TEE limitations include intracardiac echocardiography (ICE) and cardiac computed tomography (CT).13,14 ICE could potentially be performed at the time of the right heart catheterization pre-TV intervention, but its main use may be in combination with TEE during the procedure when pre-procedural TEE imaging indicates limited imaging windows. The widespread adoption of ICE has been limited by the lack of standardized imaging protocol, cost, and the learning curve.13 Cardiac CT is used to assess patient eligibility, vascular access planning, and device sizing.15,16
In summary, TEE is an essential tool in the pre-procedural evaluation of TV interventions to ascertain patient eligibility, identify the appropriate device selection, and enable procedural planning. However, TEE imaging of the TV from esophageal windows can be challenging due to the anterior and inferior location of the valve and may indicate the need for combined TEE and ICE for procedural guidance.
References
- Agricola E, Asmarats L, Maisano F, et al. Imaging for tricuspid valve repair and replacement. JACC Cardiovasc Imaging 2021;14:61-111.
- Hahn RT, Badano LP, Bartko PE, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovasc Imaging 2022;23:913-29.
- Hahn RT, Weckbach LT, Noack T, et al. Proposal for a standard echocardiographic tricuspid valve nomenclature. JACC Cardiovasc Imaging 2021;14:1299-1305.
- Hahn RT, Saric M, Faletra FF, et al. Recommended standards for the performance of transesophageal echocardiographic screening for structural heart intervention: from the American Society of Echocardiography. J Am Soc Echocardiogr 2022;35:1-76.
- Hahn RT, Kodali SK. State-of-the-art intra-procedural imaging for the mitral and tricuspid PASCAL Repair System. Eur Heart J Cardiovasc Imaging 2022;23:e94-e110.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:337-47.
- Dahou A, Ong G, Hamid N, Avenatti E, Yao J, Hahn RT. Quantifying tricuspid regurgitation severity: a comparison of proximal isovelocity surface area and novel quantitative doppler methods. JACC Cardiovasc Imaging 2019;12:560-62.
- Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F, Zoghbi WA. Imaging assessment of tricuspid regurgitation severity. JACC Cardiovasc Imaging 2019;12:469-90.
- Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging 2017;18:1342-43.
- Utsunomiya H, Harada Y, Susawa H, et al. Comprehensive evaluation of tricuspid regurgitation location and severity using vena contracta analysis: a color Doppler three-dimensional transesophageal echocardiographic study. J Am Soc Echocardiogr 2019;32:1526-37.e2.
- Lurz P, von Bardeleben RS, Weber M, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol 2021;77:229-39.
- Webb JG, Chuang AM, Meier D, et al. Transcatheter tricuspid valve replacement with the EVOQUE system: 1-year outcomes of a multicenter, first-in-human experience. JACC Cardiovasc Interv 2022;15:481-91.
- Hagemeyer D, Ali FM, Ong G, Fam NP. The role of intracardiac echocardiography in percutaneous tricuspid intervention: a new ICE age. Interv Cardiol Clin 2022;11:103-12.
- Wong I, Chui ASF, Wong CY, Chan KT, Lee MK. Complimentary role of ICE and TEE during transcatheter edge-to-edge tricuspid valve repair with TriClip G4. JACC Cardiovasc Interv 2022;15:562-63.
- Fortuni F, Hirasawa K, Bax JJ, Delgado V, Ajmone Marsan N. Multi-modality imaging for interventions in tricuspid valve disease. Front Cardiovasc Med 2021;8:638487.
- Layoun H, Schoenhagen P, Wang TKM, Puri R, Kapadia SR, Harb SC. Roles of cardiac computed tomography in guiding transcatheter tricuspid valve interventions. Curr Cardiol Rep 2021;23:114.
Clinical Topics: Noninvasive Imaging, Computed Tomography, Echocardiography/Ultrasound, Nuclear Imaging, Valvular Heart Disease
Keywords: Tricuspid Valve, Vena Cava, Superior, Echocardiography, Transesophageal, Mitral Valve, Coronary Vessels, Feasibility Studies, Jugular Veins, Learning Curve, Papillary Muscles, Cardiac Catheterization, Heart Atria, Tomography, Prolapse, Tomography, X-Ray Computed, Review Literature as Topic
< Back to Listings