Cardiorespiratory Fitness Early-Post Breast Cancer Therapy
Quick Takes
- Cardiorespiratory fitness is an independent predictor of CVD in cancer survivors – the leading non-cancer cause of death in women with HER2+ breast cancer. Identifying women with HER2+ breast cancer who are at high risk of impaired cardiorespiratory fitness at end of cancer therapy can help stratify patients for interventions such as cardio-oncology rehabilitation (CORE) to reduce future CVD risk.
- Cardiologists can use a diagnostic algorithm – based on age, global longitudinal strain, and left-sided filling pressure – to stratify survivors of HER2+ breast cancer according to their risk for having compromised functional independence (i.e., impaired cardiorespiratory fitness), and by association, higher CVD risk.
Study Questions
Among women with HER2+ breast cancer, what clinical, cardiac biomarker, and cardiac imaging measures are associated with reduced cardiorespiratory fitness (measured as VO2peak) early following breast cancer therapy, to help inform cardiovascular disease (CVD) risk stratification?1
Methods
Women with early-stage (Stage I-III) HER2+ breast cancer were prospectively recruited from Princess Margaret Cancer Center and St. Michael's Hospital, Toronto, Ontario Canada. All patients participated in the ongoing EMBRACE-MRI study or the Toronto arm of the SUCCOUR study.2 From these studies, inclusion criteria were: 1) ≥18 years of age; 2) treated with anthracyclines followed by trastuzumab with or without radiotherapy; and 3) completed all cardiac and clinical assessments before anthracyclines and after trastuzumab. Early following completion of trastuzumab therapy, we collected clinical information, systolic and diastolic echocardiographic measures (e.g., global longitudinal strain [GLS], left ventricular ejection fraction [LVEF], left-sided filling pressures [E/e']), cardiac biomarkers (i.e., B- type natriuretic peptide [BNP], high-sensitivity troponin I [hsTnI]), and VO2peak assessed via maximal cardiopulmonary exercise test (CPET) using a cycle ergometer. Regression models were used to examine the association between VO2peak and clinical, imaging, and cardiac biomarkers individually and in combination. A decision support tool using a conditional inference tree model was developed to identify individuals with a VO2peak below the threshold for independent living (defined as compromised functional independence; VO2peak <18 mL O2•kg−1•min−1),3 and by association, higher CVD risk.1,4
Results
We recruited 177 eligible women between November 2013 and January 2019. A total of 30 participants declined CPET, leaving 147 for inclusion. Mean VO2peak for the cohort was 19.1 ± 5.0 mL O2•kg−1•min−1. There were 64 (44%) participants in the entire cohort who had VO2peak <18 mL O2•kg−1•min−1 (14.9 ± 2.3 mL O2•kg−1•min−1; range 9.3-17.9 mL O2•kg−1•min−1) suggesting compromised functional independence.
In univariable analysis, absolute GLS (β coefficient: 0.72; P < 0.001), E/e' (β: -0.76; P < 0.001), cardiac medication use (β: -2.59; P = 0.003), presence of ≥1 CVD risk factor (β: -1.87; P = 0.026), age per 10 years (β: -2.08 per 10 years; P < 0.001), and history of cancer therapy related cardiac dysfunction (β: -2.60; P = 0.008) were associated with VO2peak. BNP, hsTnI, and LVEF were not associated with VO2peak. In multivariable analysis, absolute GLS (β: 0.58; P = 0.007), age per 10 years (β: −1.61 per 10 years; P = 0.001), and E/e' (β: −0.45; P = 0.038) remained associated with VO2peak.
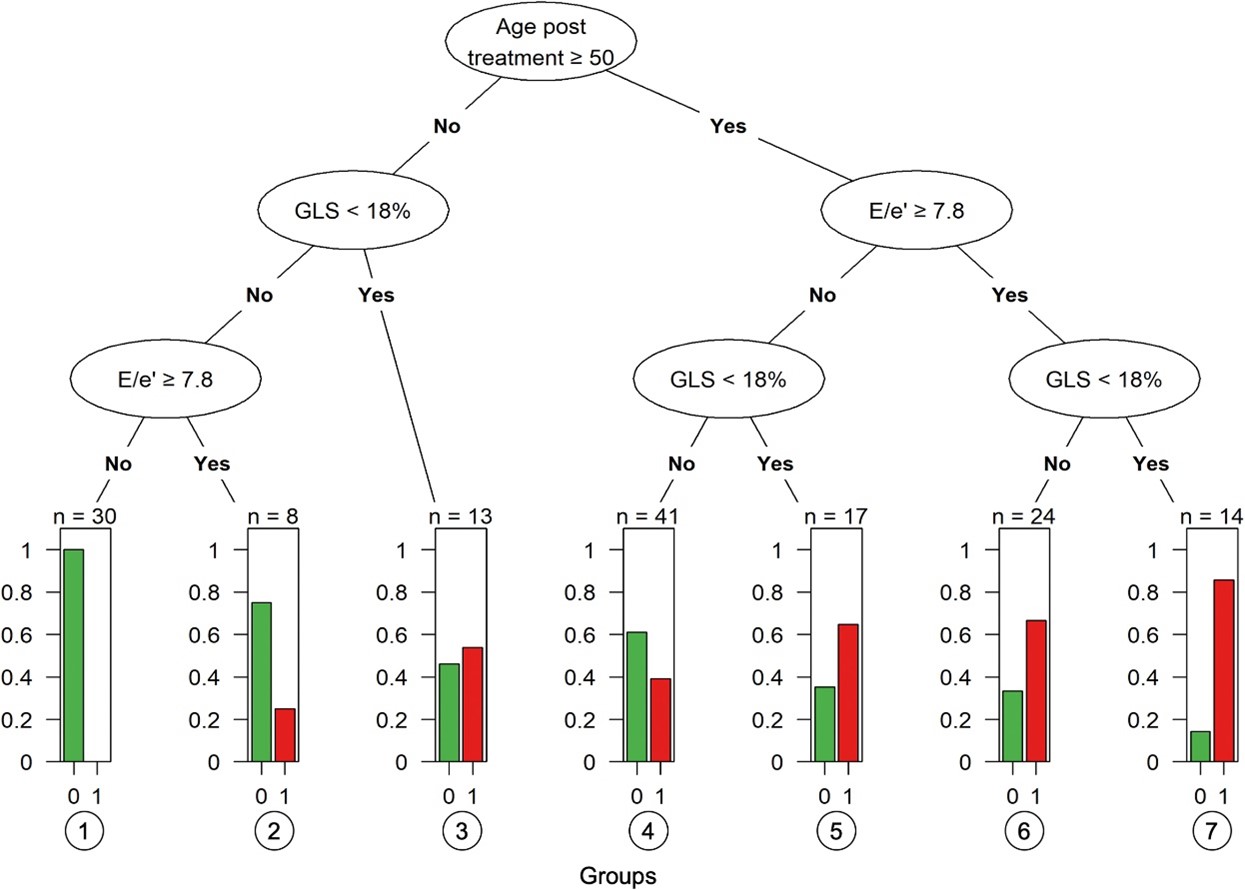
The final tree model selected age (≥50 years), GLS (<18%), and E/e' (≥7.8) as the most important variables for detecting individuals with compromised functional independence (area-under-the-curve [AUC] = 0.80; 95% confidence interval [CI]: 0.74-0.86). Participants who met all three criteria had the highest probability of compromised functional independence (85.7%), whereas those who met none of the criteria had the lowest probability (0%).
Conclusions
Age, GLS, and E/e' were independently associated with VO2peak. Using these parameters, we developed a diagnostic algorithm with good discriminatory value for identifying individuals with compromised functional independence (i.e., cardiorespiratory fitness impairment) and, by association, likely higher risk of future CVD.
Perspectives
Effective and scalable strategies are needed to mitigate cancer-related CVD risk to improve long-term survival of people diagnosed with breast cancer. However, no decision-making frameworks are available to help clinicians evaluate and stratify patients according to CVD risk at the end of cancer therapy and, subsequently, who should be prioritized for more intensive surveillance referral to key support services (e.g., cardio-oncology rehabilitation [CORE]).
From a screening perspective, this work suggests that clinicians can consider several readily available demographic and clinical factors (i.e., increased age [≥50 years], cancer therapy related cardiac dysfunction, history cardiac medications, CVD risk factors) and certain cardiac imaging parameters (i.e., E/e' [≥7.8] and GLS [<18%]) when screening patients for potentially increased risks of compromised functional independence at the end of cancer therapy (Figure 1).
Figure 1
Figure 1: Tree Diagram for Detection of Compromised Functional Independence. Bonsignore A, Marwick TH, Adams SC, et al. Clinical, echocardiographic, and biomarker associations with impaired cardiorespiratory fitness early after HER2-targeted breast cancer therapy. J Am Coll Cardiol CardioOnc 2021;3:678-91.
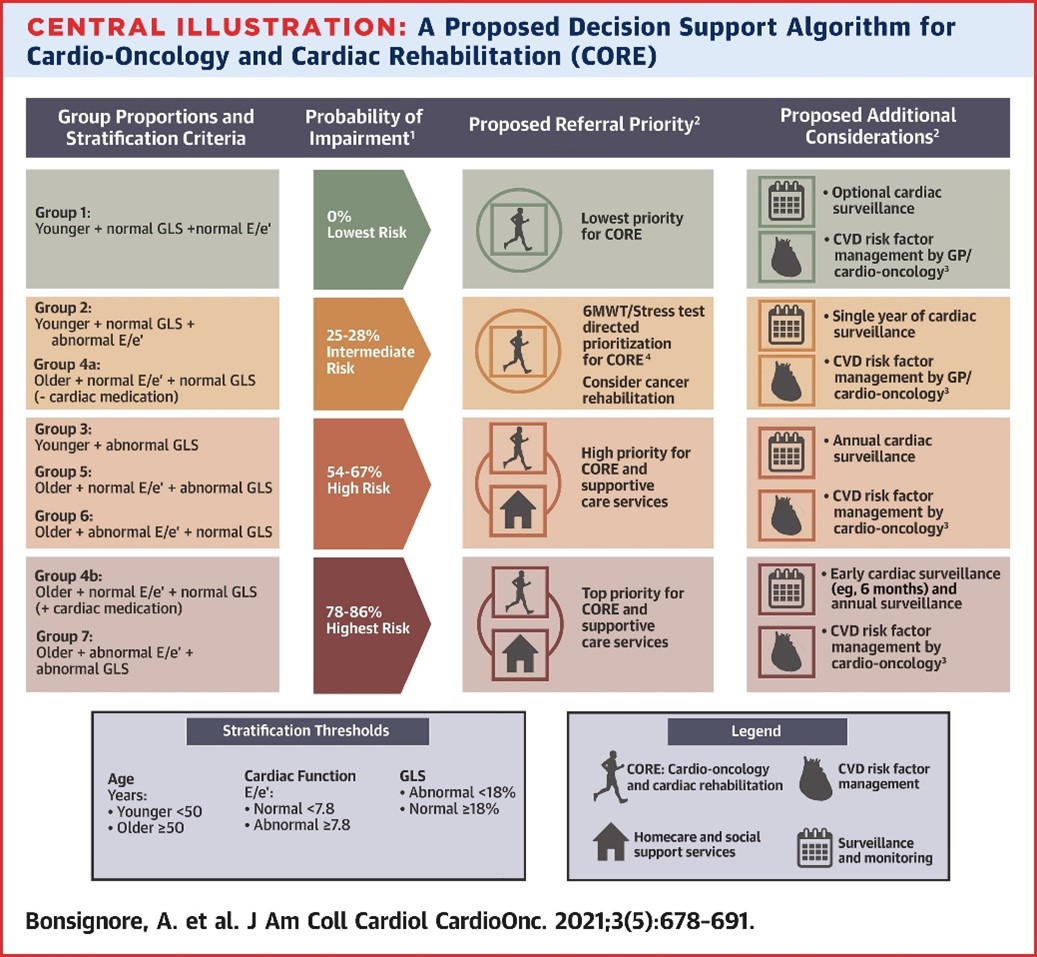
Moreover, an algorithm based on age-, GLS-, and E/e'-defined thresholds may be particularly useful in stratifying patients according to relative risk of cardiorespiratory fitness impairments at the end of treatment and subsequently increased CVD risk. These findings highlight the potential importance of assessing GLS and E/e' in women with HER2+ breast cancer given their independent associations with VO2peak. This algorithm, if validated, would provide clinicians with a scalable and cost-effective CPET alternative to identify vulnerable patients who would benefit from enhanced cardiac surveillance and CVD risk factor management in the early posttreatment period (Figure 2).
Figure 2
Figure 2: A Proposed Decision Support Algorithm for Cardio-Oncology and Cardiac Rehabilitation (CORE). Bonsignore A, Marwick TH, Adams SC, et al. Clinical, echocardiographic, and biomarker associations with impaired cardiorespiratory fitness early after HER2-targeted breast cancer therapy. J Am Coll Cardiol CardioOnc 2021;3:678-91.
The American Heart Association recently endorsed the use of multi-modal exercise-based rehabilitation programs, similar to cardiac rehabilitation (e.g., CORE), to help improve cardiovascular outcomes and reduce CVD risk in cancer survivors, including survivors of breast cancers.5 However, the blanket referral of all cancer survivors with increased CVD risk would quickly overwhelm the capacity of extant cardiac rehabilitation programs. Unfortunately, no risk stratification frameworks or tools have been developed to help clinicians decide which cancer patients and survivors should be prioritized for referral to CORE support services.6 Therefore, an algorithmic approach to screening for cardiorespiratory fitness impairments could also help clinicians identify patients who more urgently require referral to specialized support services, like CORE. If confirmed in larger trials, the use of this algorithm may be particularly beneficial in settings without access to CPET services to help risk stratify survivors of HER2+ breast cancers and may be generally useful for clinicians across all settings to facilitate faster decision making, care planning, and mobilization of appropriate support services.
References
- Groarke JD, Payne DL, Claggett B, et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes 2020;6:315-22.
- Thavendiranathan P, Negishi T, Somerset E, et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol 2021;77:392–401.
- Forman DE, Arena R, Boxer R, et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e894–918.
- Fardman A, Banschick GD, Rabia R, et al. Cardiorespiratory fitness is an independent predictor of cardiovascular morbidity and mortality and improves accuracy of prediction models. Can J Cardiol 2021;37:241–50.
- Gilchrist SC, Barac A, Ades PA, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation 2019;139:E997–1012.
- Tran M, Pesah E, Turk-Adawi K, et al. Cardiac rehabilitation availability and delivery in Canada: how does it compare with other high-income countries? Can J Cardiol 2018;34:S252–62.
Clinical Topics: Cardio-Oncology, Cardiovascular Care Team, Noninvasive Imaging, Sports and Exercise Cardiology, Novel Agents, Echocardiography/Ultrasound, Magnetic Resonance Imaging, Sports and Exercise and ECG and Stress Testing, Sports and Exercise and Imaging
Keywords: Cardiorespiratory Fitness, Stroke Volume, Breast Neoplasms, Troponin I, Exercise Test, Cardiac Rehabilitation, Cardiovascular Diseases, Cost-Benefit Analysis, American Heart Association, Area Under Curve, Cancer Survivors, Confidence Intervals, Functional Status, Ventricular Function, Left, Oxygen Consumption, Risk Factors, Heart Diseases, Risk Assessment, Echocardiography, Natriuretic Peptides, Decision Making, Anthracyclines, Trastuzumab, Algorithms, Biomarkers, Referral and Consultation, Hospitals, Magnetic Resonance Imaging
< Back to Listings