Advancements in Percutaneous Pulmonary Valve Therapeutics: Harmony & Alterra Valves, The Next Frontier
One of the most common treatment challenges in the adult with congenital heart disease (ACHD) is management of pulmonary regurgitation, particularly in the setting of tetralogy of Fallot following transannular right ventricular outflow tract (RVOT) patch. Over time, progressive pulmonary regurgitation results in right ventricular (RV) volume overload and dilation, development of systolic and diastolic dysfunction, and risk of heart failure (HF) and atrial and/or ventricular arrhythmias, accounting for significant morbidity and increased mortality risk.
Transcatheter heart valve (THV) technology was initially developed for the treatment of RVOT/pulmonic valve disease, with the first percutaneous implant in 2000.1 The balloon-expandable Melody™ valve (Medtronic, Inc.) was Food and Drug Administration (FDA) approved in 2010, and the Sapien valve (Edwards Lifesciences) in 2020 for treatment of dysfunctional RV to pulmonary artery (PA) conduits or bioprosthetic valves. However, the diameters of the surgically modified RVOT in many adolescents and adults are too large for the maximal diameters of these approved valves. To address this size limitation, two different technologies have received FDA approval in 2021 for treatment of these large, often patulous RVOTs: the Harmony™ Transcatheter Pulmonary Valve (TPV) (Medtronic Inc.) and the Alterra Adaptive Prestent system (Edwards Lifesciences).
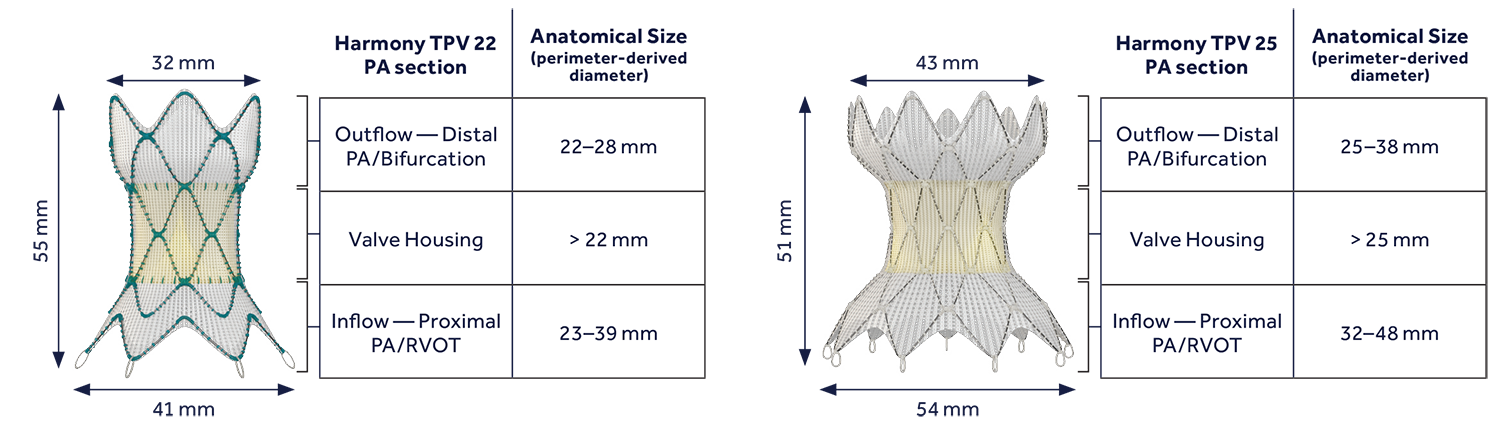
The Harmony TPV is a self-expanding valve composed of porcine pericardial leaflets mounted on a Nitinol (nickel-titanium) wire frame, with a polyester fabric covering.2,3 It is available as a 22mm valve (with an unrestrained outflow measuring 32mm, and inflow 41mm, and height 55mm) and 25mm valve (with an unrestrained outflow 43mm, inflow 54mm, and height 51mm) (Figure 1).
Figure 1
The valve is mounted on its delivery system and inserted through a 24 or 26 French long sheath. As the delivery sheath is retracted over the valve, it allows it to expand in place; it is possible to pull back, but not advance the valve during deployment. Before full expansion, the valve can be recaptured and removed if desired, but not redeployed. Rapid ventricular pacing is usually not required. It is not FDA approved for treatment of RV-PA conduits, though in rare cases patients will have suitable anatomy. The United States (US) and Canada clinical experience exceeds 500 implants. As of April 2022, however, implants are on hold as Medtronic issued a recall of the delivery system due to the risk of capsule fracture during manipulation of the delivery catheter, after a change in manufacturing processes. An updated bonding process has been established with reliable results on bench testing, and approval for return to clinical use of the delivery system is expected in Spring 2023.
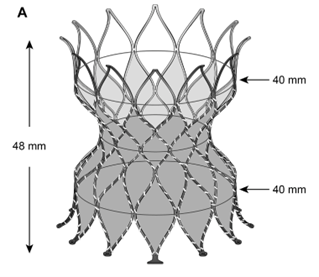
The Alterra is a self-expanding stent, also made of Nitinol, but covered with a polytetrafluoroethylene membrane.4 It has wide inflow and outflow areas (measuring 40mm; height of the system is 48mm after deployment), and a waist that is sized to allow for implantation of a 29mm Sapien S3 balloon-expandable transcatheter valve (which is made of bovine pericardium on a cobalt-chromium frame) (Figure 2).
Figure 2
The Alterra is mounted on a delivery system that can be inserted through a 22F or larger 33cm long sheath and is deployed by pulling back the delivery sheath and uncovering the device. The pre-stent can be recaptured and redeployed, though the Nitinol frame may become deformed with several attempts. The distal tip of the device is flared out to improve fixation in the distal PA. After the pre-stent system is deployed in adequate position and its delivery system removed, a 29mm Sapien S3 valve can be inserted through its own inline sheath and delivery system (26F outer diameter) and deployed inside the waist of the pre-stent. In the rare cases where there are concerns about the stability of the Alterra system, the Sapien valve implantation can be deferred until several weeks later, when the Alterra has endothelialized and risk of embolization from device interaction with the Sapien delivery system has passed.
A gated, systolic, and full diastolic phase cardiac computerized tomography (CT) angiogram is required for assessment of the anatomy for both these devices.5 To assure adequate tissue compression and device stability, the dimensions of the RVOT and main PA in systolic and late diastolic phases are evaluated; this is especially important in patients with a history of congenital pulmonary stenosis, who often have large and dynamic PAs, with increase in diameter in systole. Perimeter-derived diameters during the cardiac cycle are used to determine the degree of interference (compression) of the devices to stabilize in the RVOT; in borderline cases, assessing whether there is significant asymmetry and whether the main PA (MPA) is dynamic and distensible can be helpful. For the Harmony valve, the evaluation is focused on the diastolic phase, while for the Alterra, the systolic phase is most commonly used. Both valves depend on contact at the inflow and/or outflow to prevent valve embolization and paravalvular leak. The temporal and spatial resolution of a retrospective gated CT scan is standard of care at this time for procedural planning, though initial screening can be done with cardiac magnetic resonance imaging (MRI) to avoid radiation exposure for patients with RVOT that are definitely too large or too small for either valve system.
The valves are usually implanted in a supra-annular position (in the MPA), though they could also be implanted lower, or in an annular position. In patients with lower implantation, where the frame makes contact with the RVOT, there have been reports of ventricular tachycardia: in most cases, non-sustained short episodes that resolved in the first day or two after the procedure, but in rare instances sustained arrhythmias requiring beta-blockade have occurred (including a case of cardiac arrest requiring extracorporeal membrane oxygenation [ECMO], and a few cases requiring valve explant). With increasing worldwide experience, supra-annular implants appear preferable.
As both systems are self-expanding, and usually do not significantly distort the anatomy of the MPA, coronary compression is not thought to be a concern; assessment of the relationship between the coronaries and the RVOT is still however recommended, at least on the pre-procedural CT angiogram.
In patients with a high surgical risk and where the RVOT and MPA are too large to offer a suitable landing zone, a hybrid procedure, with sternotomy and banding of the MPA, has been reported.6 Prior to Harmony valve approval, hybrid procedures with MPA banding to allow a Sapien S3 valve implantation had also been performed; the band however has to be precisely sized for the balloon-expandable valve, whereas the larger Harmony and Alterra devices allow greater flexibility in the dimensions of the MPA.
The longevity of both systems is expected to be comparable to balloon-expandable transcatheter pulmonary valves of similar diameters;7 it is likely to be dependent on patient age, size, somatic growth, and risk of endocarditis. Encouraging data is available on 5-year follow-up for the initial cohort of 20 patients who underwent Harmony implantation in the Early Feasibility study.7 Antiplatelet therapy is recommended after implantation, but it is not yet known whether aspirin, thienopyridine, dual antiplatelet therapy, or even short-term anticoagulation, might lead to optimal valve longevity.8,9 A 29mm Sapien S3 valve could be implanted in a valve-in-valve fashion in either system if needed.10 There is yet little experience with surgical explants of either system; it is likely to require more time for dissection, and MPA patching. In many ACHD patients who will need to undergo multiple interventional and surgical procedures over the course of their lifetimes, planning the order of transcatheter versus surgical valve replacement is crucial as a multidisciplinary team discussion.
Both systems are expected to have a steep learning curve, both regarding patient selection, procedural techniques, and follow-up. A post-market surveillance program and multi-institutional independent registry will be critical to identify best practices. While most implanted patients are expected to be included in the IMPACT Registry® section on transcatheter pulmonary valves, additional data focusing on technical implant details as well as follow-up will be important.
Both new systems, as well as other valves designed for large native RVOTs around the world, are part of a revolution in the treatment of children and adults with CHD.
References
- Bonhoeffer P, Boudjemline Y, Qureshi SA, et al. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol 2002;39:1664-69.
- Bergersen L, Benson LN, Gillespie MJ, et al. Harmony Feasibility trial: acute and short-term outcomes with a self-expanding transcatheter pulmonary valve. JACC Cardiovasc Interv 2017;10:1763-73.
- Benson LN, Gillespie MJ, Bergersen L, et al. Three-year outcomes from the Harmony native outflow tract Early Feasibility study. Circ Cardiovasc Interv 2020;13:e008320.
- Shahanavaz S, Balzer D, Babaliaros V, et al. Alterra Adaptive Prestent and SAPIEN 3 THV for congenital pulmonic valve dysfunction: an Early Feasibility study. JACC Cardiovasc Interv 2020;13:2510-24.
- Gillespie MJ, Benson LN, Bergersen L, et al. Patient selection process for the Harmony transcatheter pulmonary valve Early Feasibility Study. Am J Cardiol 2017;120:1387-92.
- Phillips AB, Nevin P, Shah A, Olshove V, Garg R, Zahn EM. Development of a novel hybrid strategy for transcatheter pulmonary valve placement in patients following transannular patch repair of tetralogy of fallot. Catheter Cardiovasc Interv 2016;87:403-10.
- Gillespie MJ, Bergersen L, Benson LN, Weng S, Cheatham JP. 5-Year outcomes from the Harmony native outflow tract Early Feasibility study. JACC Cardiovasc Interv 2021;14:816-17.
- Shibbani K, Garg R, Zahn EM, McLennan D. Aspirin use and transcatheter pulmonary valve replacement, the need for consistency. Pediatr Cardiol 2021;42:1640-46.
- Egbe AC, Connolly HM, Miranda WR, Dearani JA, Schaff HV. Outcomes of bioprosthetic valves in the pulmonary position in adults With congenital heart disease. Ann Thorac Surg 2019;108:1410-15.
- Anderson JH, Taggart NW, Hagler D, Cabalka A. Valve-in-frame implantation: SAPIEN in Harmony. JACC Cardiovasc Interv 2022;15:e63-e64.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Valvular Heart Disease, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, Cardiac Surgery and VHD, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Imaging, CHD and Pediatrics and Interventions, CHD and Pediatrics and Quality Improvement, Interventions and Imaging, Interventions and Structural Heart Disease, Angiography, Magnetic Resonance Imaging, Nuclear Imaging
Keywords: Tetralogy of Fallot, Polytetrafluoroethylene, Dilatation, United States Food and Drug Administration, Pulmonary Valve Insufficiency, Heart Defects, Congenital, Pericardium, Stents, Catheters, Arrhythmias, Cardiac, Morbidity, Sternotomy, Heart Valve Prosthesis, Retrospective Studies, Systole, Extracorporeal Membrane Oxygenation, Follow-Up Studies, Transcatheter Aortic Valve Replacement, Pulmonary Valve Stenosis, Endocarditis, Angiography, Magnetic Resonance Imaging, Registries, Patient Care Team, Anticoagulants, Thienopyridines, Tachycardia
< Back to Listings