Past, Present, and Future of Transcatheter Pulmonary Valves
Quick Takes
- Balloon-expandable valves are critically important but offer a solution only to a minority of pediatric and adult patients with congenital heart disease needing transcatheter pulmonary valve replacement.
- Newer self-expanding systems are available for native right ventricular outflow tracts that can encompass more patients with congenital heart disease.
- New valves are in development that can be deployed and serially dilated in smaller patients.
Almost one-quarter of a century has passed since the first Melody™ transcatheter pulmonary valve (Medtronic, Minneapolis, MN) was implanted.1 Since then, there has been a significant increase in options for transcatheter valve replacement, which in general, can be divided into balloon-expandable and self-expanding valves. Balloon-expandable valves offer more controlled deployment and precise positioning that can be critical in certain anatomical situations, whereas self-expanding valves can conform better to irregular vessel shapes and can be deployed in much larger diameter vessels.
At the time of this publication, the options for transcatheter pulmonary valve replacement (TPVR) consist of the following: Melody valve, Sapien 3™ (Edwards Lifesciences, Irvine, CA), Harmony™ (Medtronic, Minneapolis, MN), Pulsta™ (Taewoong Medical Co., Gyeonggi-do, South Korea), Venus P-Valve™ (Venus Medtech, Hangzhou, China), and the Med-Zenith PT-Valve™ (Med-Zenith, Beijing, China). Of these, only the first three are Food and Drug Administration (FDA) approved for use in the United States (Figure 1).
Figure 1: Characteristics of the Valves/Prestent Available in the US Market
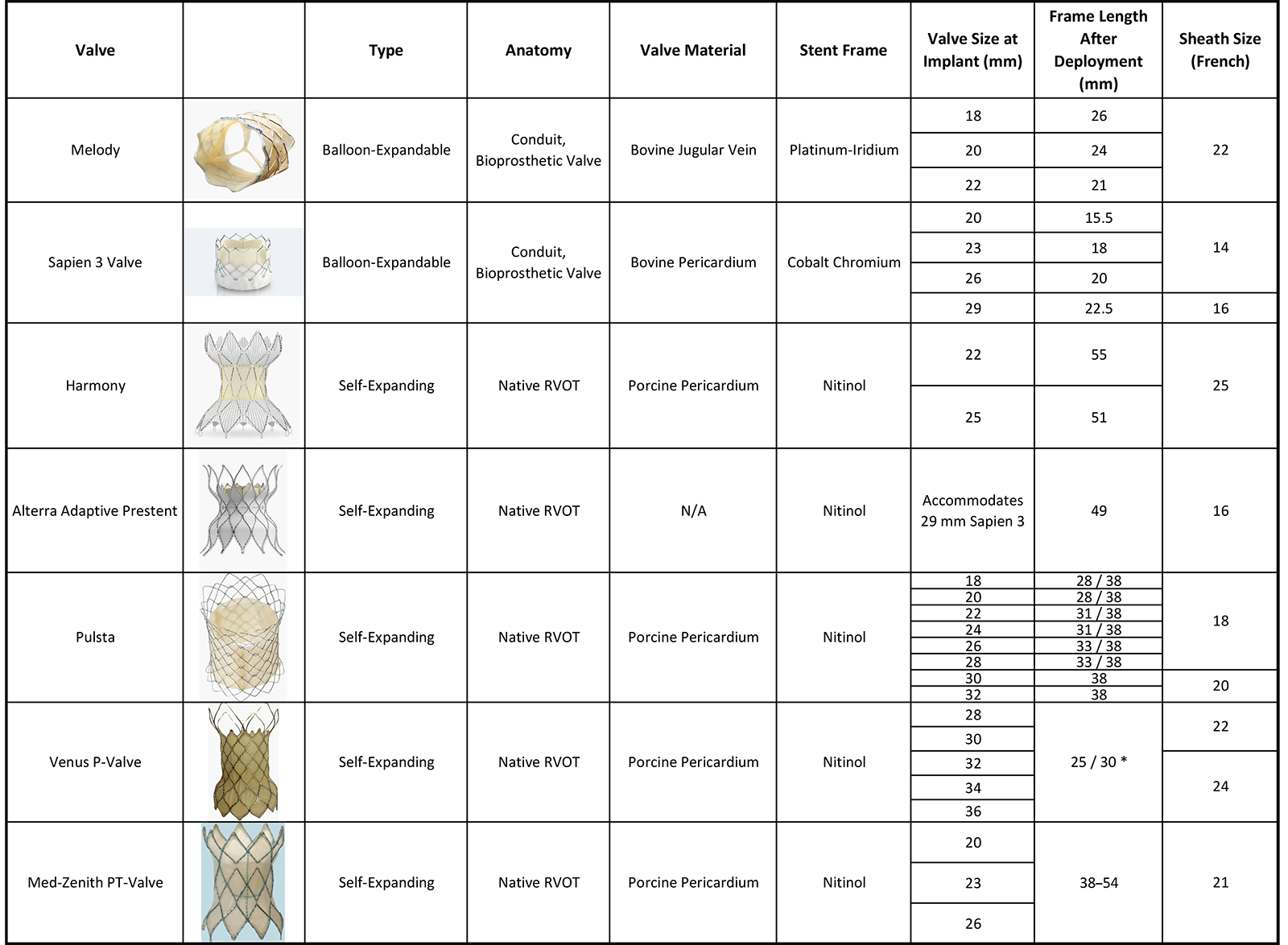
Adapted with permission from Patel ND, Levi DS, Cheatham JP, Qureshi SA, Shahanavaz S, Zahn EM. Transcatheter pulmonary valve replacement: a review of current valve technologies. J Soc Cardiovasc Angiogr Interv. 2022;1(6):100452. Published 2022 Sep 16. doi:10.1016/j.jscai.2022.100452.
Balloon-Expandable Valves
The Melody valve is made up of a valved segment of a jugular bovine vein that is sutured onto a platinum-iridium stent frame. The Melody valve is delivered using an Ensemble™ II transcatheter delivery system. Three sizes of the delivery system exist: 18, 20, and 22 mm, which refer to the diameter of the balloon onto which the valve is crimped.
The Sapien valve is built for aortic valve replacement but has been successfully adapted for TPVR. The Sapien valve is made from bovine pericardium that is shaped into three leaflets and mounted on a cobalt-chromium frame.1 The Sapien 3 is available as a 20-, 23-, 26-, and 29-mm diameter. It can be delivered either using a Commander delivery system or the Edwards Sapien 3 pulmonic delivery system.
The Sapien valve is shorter, but the Melody valve can be deployed in smaller conduit sizes with successful deployment reported in conduits <16 mm for the Melody valve.2
Self-Expanding Systems
In approximately 80% of patients with congenital heart disease,1 the right ventricular outflow tract (RVOT) is too large to accommodate balloon-expandable valves. Self-expanding valves, which are designed to be deployed in larger diameter landing zones, have therefore allowed implantation in a dilated RVOT.
The Harmony valve received FDA approval for TPVR in the native RVOT in 2021.3 The valve frame is made from six rows of nitinol wire struts, onto which a polyester cloth is hand sewn. The valve itself is made from porcine pericardium. There are two valve sizes available, the TPV22 and the TPV25, in reference to the size of the valve housed within the stent frame.
The Alterra™ adaptive prestent system (Edwards Lifesciences, Irvine, CA) was the second self-expanding RVOT system to receive FDA approval in the United States. The Alterra prestent is composed of a nitinol backbone with polytetrafluoroethylene covering on all but the distal most row of cells. The proximal and distal tips of the Alterra prestent are curved to ensure better stability. The prestent itself is not valved; rather, it acts as a landing zone for a 29 mm Sapien S3 valve that is delivered separately.4 A gated computed tomography scan is required to assess feasibility of the Harmony valve and the Alterra prestent system.
Indications for TPVR Valves
TPVR is indicated in symptomatic patients with significant pulmonary regurgitation or stenosis. If asymptomatic RV dysfunction is present, the degree of dilatation, stenosis, and regurgitation plays a role in decision-making. However, more nuanced guidelines that identify ideal timing of valve replacement are lacking, leading to variability in TPVR timing across the field.
Indeed, there is an abundance of literature with various reported criteria for TPVR.5 For the Melody valve, the Investigational Device Exemption trial included patients with a conduit or a surgical valve with New York Heart Association (NYHA) heart failure class II, III, or IV who had moderate or severe regurgitation or a mean RVOT gradient ≥35 mm Hg (NYHA class I patients were included only if they had severe pulmonary regurgitation or a mean gradient across the RVOT of ≥40 mm Hg).6
The COMPASSION (Congenital Multicenter Trial of Pulmonic Valve Regurgitation Studying the SAPIEN Transcatheter Heart Valve) trial for Sapien valve TPVR included patients who have a conduit or a surgical valve with grade ≥3+ regurgitation by echocardiography or ≥40% regurgitation by magnetic resonance imaging (MRI) or mean gradient across the RVOT of ≥35 mm Hg.7
The Harmony Native TPV EFS (Early Feasibility Study) included patients who had regurgitation that was either severe by echocardiography or was ≥30% by MRI, and who were either symptomatic or had RV end-diastolic volume index ≥150 mL/m2.8
Although these documents provide some framework for clinicians, they are not universally adopted or endorsed, and interventions beyond the recommendations listed earlier are frequent.
Outcomes for TPVR
Long-term outcomes for the Melody valve are encouraging and showed 90% freedom from mortality, 60% freedom from reintervention, and 79% freedom from reoperation.6 Freedom from valve dysfunction at 10 years was 53%, and an annualized rate of endocarditis was 2%. For the Sapien valve, 3-year follow-up showed freedom from mortality of 98.4%, freedom from reintervention of 93.7%, and freedom from endocarditis of 97.1%.7
Direct long-term outcome comparison between the Sapien and Melody valves showed that the freedom from secondary pulmonary valve replacement at 10 years was 50.4% for the Melody valve and 82.2% for the Sapien valve.9 The incidence of infective endocarditis was 5.5/100 patient-years with the Melody valve and 0.2/100 patient-years for the Sapien valve.
Harmony valve 5-year follow-up showed 90% freedom from surgical intervention, 85% freedom from catheter-based interventions, and 95% freedom from mortality.10 At 5 years, regurgitation was mild or less in 100% of patients and the mean gradient across the valve was 15.5 mm Hg.
The Alterra early feasibility study showed 100% successful device placement with no dysfunction reported at 6 months. Long-term data are currently lacking for the Alterra prestent system.4
Future Landscape
Options for balloon-expandable valves in the United States will likely increase as the Pulsta, Venus P-Valve, and the Med-Zenith PT-Valve undergo review for FDA approval. One patient population that is still lacking any TPVR options, however, is very young children who are too small to undergo valve replacement with any of the currently available devices. This is an area of active research with several such valves in various stages of development including the Autus™ valve (Autus Valve Technologies, Boston, MA), IRIS™ valve, and PolyVascular™ valve (Polyvascular Inc., Houston, TX).
References
- Patel ND, Levi DS, Cheatham JP, Qureshi SA, Shahanavaz S, Zahn EM. Transcatheter Pulmonary Valve Replacement: A review of current valve technologies. J Soc Cardiovasc Angiogr Interv. 2022;1(6):100452. Published 2022 Sep 16. doi:10.1016/j.jscai.2022.100452
- Tengler A, Ulrich S, Fischer M, et al. Rationale and feasibility of transcatheter pulmonary valve implantation in small conduits with the Edwards Sapien valves. Int J Cardiol. 2021;325:45-50. doi:10.1016/j.ijcard.2020.10.017
- Chau AK. Transcatheter pulmonary valve replacement in congenital heart diseases. Pediatr Investig. 2022;6(4):280-290. Published 2022 Dec 5. doi:10.1002/ped4.12359
- Shahanavaz S, Balzer D, Babaliaros V, et al. Alterra adaptive prestent and SAPIEN 3 THV for congenital pulmonic valve dysfunction: an early feasibility study. JACC Cardiovasc Interv. 2020;13(21):2510-2524. doi:10.1016/j.jcin.2020.06.039
- Han BK, Garcia S, Aboulhosn J, et al. Technical recommendations for computed tomography guidance of intervention in the right ventricular outflow tract: Native RVOT, conduits and bioprosthetic valves: a white paper of the Society of Cardiovascular Computed Tomography (SCCT), Congenital Heart Surgeons' Society (CHSS), and Society for Cardiovascular Angiography & Interventions (SCAI). J Cardiovasc Comput Tomogr. 2024;18(1):75-99. doi:10.1016/j.jcct.2023.06.005
- Jones TK, McElhinney DB, Vincent JA, et al. Long-term outcomes after melody transcatheter pulmonary valve replacement in the US Investigational Device Exemption Trial. Circ Cardiovasc Interv. 2022;15(1):e010852. doi:10.1161/CIRCINTERVENTIONS.121.010852 J
- Kenny D, Rhodes JF, Fleming GA, et al. 3-year outcomes of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. JACC Cardiovasc Interv. 2018;11(19):1920-1929. doi:10.1016/j.jcin.2018.06.001
- Benson LN, Gillespie MJ, Bergersen L, et al. Three-year outcomes from the Harmony Native Outflow Tract Early Feasibility Study. Circ Cardiovasc Interv. 2020;13(1):e008320. doi:10.1161/CIRCINTERVENTIONS.119.008320
- Houeijeh A, Batteux C, Karsenty C, et al. Long-term outcomes of transcatheter pulmonary valve implantation with Melody and SAPIEN valves. Int J Cardiol. 2023;370:156-166. doi:10.1016/j.ijcard.2022.10.141
- Gillespie MJ, Bergersen L, Benson LN, Weng S, Cheatham JP. 5-year outcomes from the Harmony Native Outflow Tract Early Feasibility Study. JACC Cardiovasc Interv. 2021;14(7):816-817. doi:10.1016/j.jcin.2021.01.046
Clinical Topics: Congenital Heart Disease and Pediatric Cardiology, Congenital Heart Disease, Invasive Cardiovascular Angiography and Intervention
Keywords: Pulmonary Valve, Heart Defects, Congenital
