Deep Learning Model Accurately Identifies MV Prolapse From TTEs
A deep learning model accurately identified mitral valve prolapse (MVP) from transthoracic echocardiograms (TTE), and its predictions were associated with clinical endpoints such as mitral regurgitation (MR) and future mitral valve repair or replacement (MVR), thus it could automate the diagnosis of MVP, according to a study published Sept. 30 in JACC: Cardiovascular Imaging.
Mostafa A. Al-Alusi, MD, MS, et al., developed DROID-MVP (Dimensional Reconstruction of Imaging Data–Mitral Valve Prolapse), a deep learning model trained and validated using 1,043,893 TTE videos including 48,829 studies from 16,902 adult cardiology patients (mean age, 61 years; 38% women) at Massachusetts General Hospital (MGH). The model was externally validated in primary care patient cohorts from MGH (n=8,888) and Brigham and Women's Hospital (BWH) (n=257).
Results showed that 5% had MVP in the internal cohort (derivation sample) vs. 2% in the combined external cohort. The overall study-level classification accuracy of DROID-MVP was 98% in the internal validation sample; external validation sample accuracy was 99% with MGH and 92% with BWH cohorts.
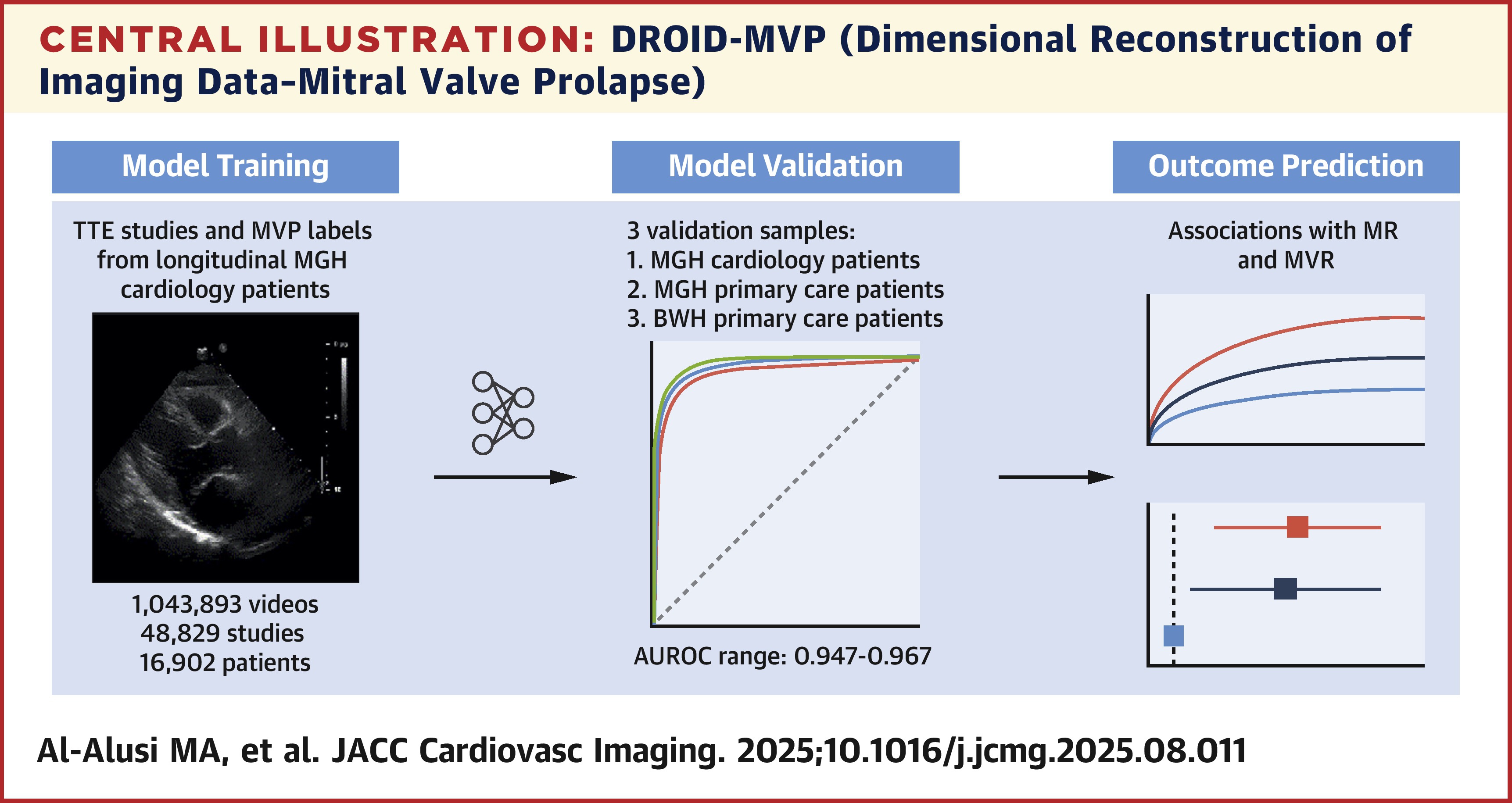
Findings also revealed that DROID-MVP accurately identified MVP across all cohorts. The MGH cardiology internal validation cohort had an area under the receiver operating characteristic curve (AUROC) of 0.947, average precision (AP) of 0.682 and prevalence of 0.036. The MGH external validation cohort had an AUROC of 0.964, AP of 0.651 and prevalence of 0.022, whereas the BWH external validation cohort had an AUROC of 0.968, AP of 0.774 and prevalence of 0.113.
Notably, higher (>0.67) vs. lower (<0.33) DROID-MVP scores were linked to moderate or severe MR (adjusted odds ratio, 2.0; p=0.030) and a greater incidence of future MVR (adjusted hazard ratio, 3.7; p=0.004), "suggesting that DROID-MVP captures information about the clinical significance of MVP that may be useful in risk stratification," write Al-Alusi and colleagues.
In an accompanying editorial comment, David Messika-Zeitoun, MD, PhD, et al., write that the study authors "deserve recognition for this pioneering effort to apply [artificial intelligence] to MVP identification in TTE." However, they note several limitations, stating that "additional work is required to refine the accuracy of MVP detection and to validate these findings in large, well-annotated, and diverse data sets.... Only then can such algorithms be considered sufficiently robust for integration into routine clinical practice within echocardiography laboratories."
Clinical Topics: Noninvasive Imaging, Echocardiography/Ultrasound
Keywords: Mitral Valve Prolapse, Artificial Intelligence, Deep Learning, Echocardiography
< Back to Listings
