FDA Expands TAVR Indication to Low-Risk Patients
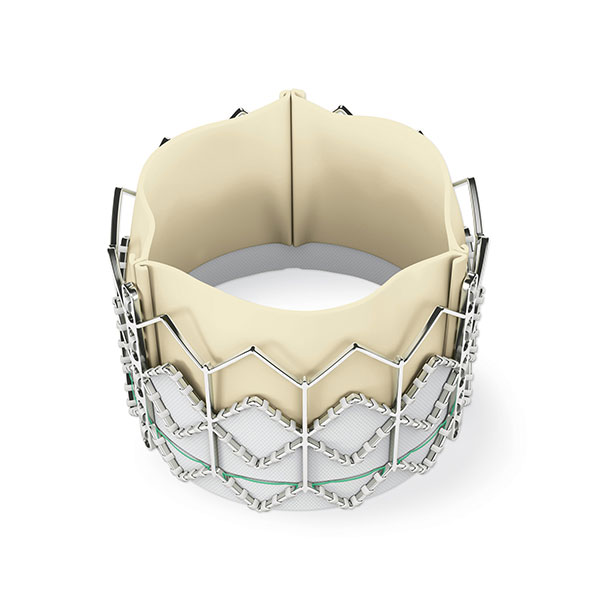
The U.S. Food and Drug Administration (FDA) has approved an expanded indication for several transcatheter heart valves (Sapien 3, Sapien 3 Ultra, CoreValve Evolut R and CoreValve Evolut PRO) to include patients with severe aortic valve stenosis at low surgical risk.
The FDA is the first medical products regulatory body in the world to expand the indication for TAVR to this patient population.
As part of the approval process, manufacturers are required to continue to follow patients enrolled in their randomized studies for 10 years to further monitor safety and effectiveness, including long-term valve durability. Read more.
