Can We Safely Provide Cancer-Directed Therapies to Patients With LVADs and Active Malignancies?
Quick Takes
- In the largest-to-date retrospective cohort to describe outcomes in patients with left ventricular assist device (LVAD) and active cancer,1 we found no statistical difference in unadjusted survival between patients with malignancy and their age-, sex-, cardiomyopathy type-, implant strategy-, and INTERMACS profile-matched comparators without cancer. Cancer diagnosis as a time-varying covariate was associated with a statistically significant increase in death.
- Among patients with diagnosis of early-stage (non-metastatic) cancer after LVAD, most were treated with curative intent. The incidence of severe complications (LVAD thrombosis, stroke, and infection) was not significantly different between the groups except for gastrointestinal bleed, which was less frequent in patients with active cancer.
- This study provides initial safety data for the management of patients with active cancer and LVAD and a provides a platform for multidisciplinary decision making that is relevant for medical and radiation oncology, surgical oncology, cardiac surgery, and all heart failure (HF) and palliative team members.
Study Objectives
Limited data exist to guide oncology and cardiology decision making in patients with an LVAD and concurrent active malignancy. The goal of this study was to investigate cancer treatment approaches (curative and palliative), incidence of complications, and survival among patients with active malignancy on LVAD support.
Methods
This retrospective, matched-control cohort study was conducted in two tertiary-care centers with high-volume advanced HF, oncology, and cardio-oncology programs. LVAD databases were reviewed to identify patients with a cancer diagnosis at the time of or after LVAD implantation. Exact 3:1 matching (stratified by site) of non-cancer comparators to patients with active cancer was performed based on the age (±3 years), sex, implant intention (destination therapy, bridge to transplant, or bridge to candidacy), cardiomyopathy etiology (ischemic and/or nonischemic), and INTERMACS profile. HF history, LVAD implantation data, cancer stage and diagnosis, details of cancer-directed therapy (systemic therapy, radiation, and surgery), and complications (LVAD thrombosis, stroke, bleeding, and infections) were identified through individual patient's records. Hospitalizations and death were also recorded.
LVAD implant time was treated as time zero, and incidence rates for complications were calculated as time to first event, with follow-up until death or end of the study. Kaplan-Meier curves were generated starting at the time of LVAD implant to death. Patients were censored at LVAD explant or end of study. Cox proportional hazard regression analysis was used to compare mortality between patients with LVAD with and without cancer.
Results
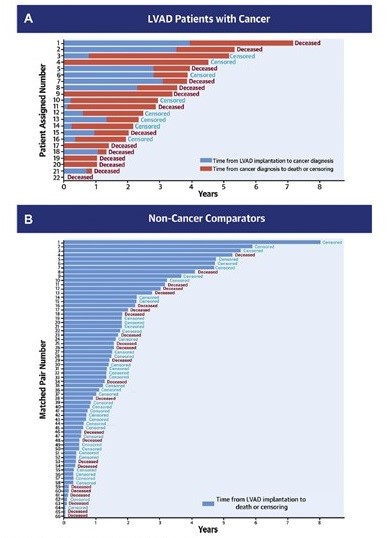
Of 1,123 patients who underwent LVAD implantation, 22 patients had active cancer and concurrent LVAD. Of those patients, 6 had a cancer diagnosis prior to LVAD implantation (with LVAD as bridge to cancer treatment), and 16 were diagnosed while on LVAD support. The matched non-cancer group consisted of 66 patients matched based on the noted variables. Among the 22 patients with LVAD and active malignancy, 73% were male, and 50% identified as Black, 41% as Caucasian and 9% as Asian (Table 1). The most common etiologies of cardiomyopathy were idiopathic and ischemic, followed by chemotherapy induced. The most common types of cancer were prostate, renal, and hematologic malignancies. Of 14 patients diagnosed with early-stage cancer, 13 received curative intent treatment, and 1 patient received no therapy. Of 8 patients with locally advanced or metastatic disease, 6 were treated with diverse palliative regimens, and 2 did not receive cancer therapy. Fifty-five percent of patients underwent surgery as a part of their cancer therapy, 50% received systemic therapy, and 23% radiation therapy. Individual patient diagnoses, cancer treatment received, and complications are presented in Table 2, with the corresponding individual patient survival shown in Figure 1. There were no significant differences in the complication event rates between patient groups for pump thrombosis (p = 0.858), ischemic stroke (p = 0.999), hemorrhagic stroke (p = 0.461), LVAD-related infection (p = 0.395), or non-LVAD-related infection (p = 0.090). Gastrointestinal bleeding was less frequent among patients with cancer compared with matched comparators (p = 0.016).
Table 1: Demographic Characteristics of Patients With LVAD With Active Malignancy and Matched Controls
| Characteristic | Patients With Active Malignancy n = 22 |
Matched Control Patients n = 66 |
| Sex – n (%) Male Female |
16 (73) 6 (27) |
48 (73) 18 (27) |
| Race – n (%) Black/African American Caucasian Asian American Indian Hispanic |
11 (50) 9 (41) 2 (9) 0 0 |
28 (41) 35 (53) 1 (2) 1 (2) 1 (2) |
| Cardiomyopathy – n (%) Idiopathic Ischemic Chemotherapy induced Other* |
8 (36) 8 (36) 4 (18) 2 (10) |
32 (48) 27 (41) 0 7 (11) |
| Median Age at LVAD implant – n (range) | 62 (41-73) | 62 (41-76) |
| Goal of LVAD implant – n (%) Destination therapy Bridge to transplant Bridge to decision |
14 (64) 6 (27) 2 (9) |
42 (64) 15 (23) 9 (13) |
| Type of LVAD – n (%) HeartMate 2 Medtronic Heartware Ventricular Assist Device HeartMate 3 |
11 (50) 7 (32) 4 (18) |
23 (35) 26 (39) 17 (26) |
| * Sarcoid (3), Hypertrophic (2), myocarditis, familial, valvular heart disease, and post-viral. | ||
Table 2: Cancer Diagnosis, Cancer Treatments Received, and Complications Among Patients With LVAD With Malignancy
| Cancer Diagnosis | Cancer Therapies | Complications | ||||
| Surgery (n = 12) |
Systemic Therapy (n = 11) |
Radiation Therapy (n = 5) |
Bleeding (n = 10) |
Thrombosis (n = 7) |
Infection (n = 9) |
|
| Prostate | Radical prostatectomy | Leuprolide | Bacteremia (driveline infection) |
|||
| Prostate | Trans-ureteral resection of the prostate | Leuprolide | Ischemic cerebrovascular accident | Bacteremia (driveline infection) | ||
| Prostate | Leuprolide | 7920 Gy | Upper gastrointestinal bleed | Pump thrombosis | ||
| Prostate | Leuprolide | Ischemic cerebrovascular accident | ||||
| Prostate | Trans-ureteral resection of the prostate | Leuprolide | Intracerebral hemorrhage | Pump thrombosis | Osteomyelitis | |
| Renal cell carcinoma | 5000 Gy | |||||
| Renal cell carcinoma | Nephrectomy | Bleeding from surgical site | Abdominal wall infection (related to driveline) | |||
| Renal cell carcinoma | Nephrectomy | |||||
| Renal cell carcinoma | Nephrectomy | Nivolumab, then ipilimumab nivolumab | 2000 Gy | |||
| Acute myeloid leukemia | Fludarabine, cytarabine, idarubicin | Upper, lower gastrointestinal bleed | Cellulitis and pneumonia | |||
| Chronic lymphocytic leukemia | Anemia | |||||
| Multiple myeloma | Cyclophosphamide, bortezomib, and dexamethasone, then ixazomib and pomalidomide | |||||
| Breast | Nab-paclitaxel and atezolizumab | Sepsis | ||||
| Breast | Lumpectomy | Anastrazole | 6040 Gy | |||
| Non-small cell lung cancer | Anemia | |||||
| Non-small cell lung cancer | Carboplatin, paclitaxel, pembrolizumab | 5000 Gy | Sepsis | |||
| Bladder | Trans-ureteral resection of bladder tumor | Hematuria | Pump thrombosis | |||
| Bladder | Trans-ureteral resection of bladder tumor | Hematuria | ||||
| Neuroendocrine tumor of the pancreas | Pancreaticoduodenectomy | Bacteremia (driveline infection) | ||||
| Neuroendocrine tumor of the colon | Sigmoidectomy | Lower gastrointestinal bleed | ||||
| Liposarcoma | Embolic cerebrovascular accident | Sternal wound infection | ||||
| Cervical | Hysterectomy and bilateral salpingo-oophoerectomy | Vaginal bleed | Pump thrombosis | |||
Figure 1: Survival After LVAD Implantation in Patients With Cancer and Non-Cancer Comparators
Kaplan-Meier survival curves showed no statistically significant difference in unadjusted survival in patients with LVAD with cancer and matched non-cancer comparators (log-rank p = 0.99), with median survival estimate from the time of LVAD placement of 3.53 and 3.03 years, respectively. In Cox proportional hazard models, however, cancer diagnosis as a time-varying variable was associated with a statistically significant increase in death (hazard ratio 2.05; p = 0.04).
Conclusions
This study provides initial data on cancer-directed treatment, complications, and outcomes for patients with concurrent LVAD and active malignancy.
Perspective
Cancer and heart disease remain the leading causes of death in the United States.2 With the increasing prevalence of HF and cancer, there is a growing population at risk of developing these disease states concurrently, with limited data to guide oncology and advanced HF care.
When deciding about the intent of cancer-directed therapy (curative vs. palliative) among patients with LVAD, the oncologists and cardiologists need to consider of complications (such as bleeding, infections, and thrombosis) as well as mortality. In our analysis, there was no significant difference in unadjusted survival estimates from the time of LVAD placement between 22 patients with cancer (3.53 years) and 66 matched non-cancer comparators (3.03 years). Of interest, patients with cancer had less gastrointestinal bleeding compared with their matched non-cancer comparators (p = 0.016), whereas other complications were not significantly different.
Our cohort also included 6 patients who underwent LVAD implantation as part of the bridge-to-cancer treatment strategy, thus challenging the paradigm of an active cancer diagnosis being a contraindication for LVAD placement. Although the literature supporting this approach remains limited, we anticipate that the need for mechanical circulatory support will grow in the future with increased effectiveness of novel cancer therapies.3,4
In summary, for patients with active malignancy on LVAD support, our study provides a framework for future prospective research regarding the intent and choice of cancer-directed therapies and for clinical, multidisciplinary complication management. Our results also point to the need to prospectively examine the candidacy criteria for the use of mechanical assist devices in patients with active malignancy to reflect contemporary oncology care.
References
- Schlam I, Lee AY, Li S, et al. Left Ventricular Assist Devices in Patients With Active Malignancies. JACC CardioOncol 2021;3:305-15.
- Leading Causes of Death (Centers for Disease Control and Prevention website). March 1, 2021. Available at https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed June 25, 2021.
- Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving Indications, Outcomes, and Scientific Partnerships. Ann Thorac Surg 2019;107:341-53.
- Molina EJ, Shah P, Kiernan MS, et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg 2021;111:778-92.
Clinical Topics: Cardio-Oncology, Cardiovascular Care Team, Heart Failure and Cardiomyopathies, Vascular Medicine
Keywords: Cohort Studies, Kaplan-Meier Estimate, Proportional Hazards Models, African Americans, Tertiary Care Centers, Retrospective Studies, Cause of Death, Follow-Up Studies, Brain Ischemia, Hemorrhagic Stroke, Stroke, Oncologists, Neoplasms, Heart Diseases, Contraindications, Thrombosis, Cardiomyopathies, Hospitalization, Hematologic Neoplasms, Gastrointestinal Hemorrhage, Risk Factors, Antineoplastic Agents, Ischemic Stroke, Decision Making, Radiation, Cardio-oncology, Cardiotoxicity
< Back to Listings