Identifying the Roadblocks to Engagement of Women in Clinical Trials
Quick Takes
- Women are consistently under-represented in cardiovascular trials.
- While there are barriers for women at every level of trial development, there are specific actionable items that can be taken to improve enrollment.
- Increasing enrollment, representation, and retention of women in all cardiovascular trials from pharmaceutical to procedural is necessary to provide better data-driven care for patients.
Introduction
Cardiovascular disease (CVD) is the leading cause of death among women.1 Despite the high and growing burden of disease, women continue to be under-represented in cardiovascular clinical trials.2 Examination of the participation-to-prevalence ratio (PPR) by Poon et al. has established a benchmark for quantifying this unequal representation. An ideal PPR between 0.8 to 1.2 indicates that the percentage of women included in studies is comparable to the percentage and prevalence of a particular disease burden in the greater society.3
Jin et al. found that in trials between 2010-2017, women were under-represented in trials for coronary artery disease (CAD), heart failure (HF), and acute coronary syndrome (ACS) with PPRs of 0.67, 0.48, and 0.66, respectively.4 Women had lower overall PPRs in trials pertaining to device therapies and procedures.4 Pulmonary artery hypertension trials are the outlier with a higher expected representation in trials with a PPR of 1.4.
Such discrepancies in representation impede clinical practice. As women have increasing burden of CVD and require more therapy, current prescribing patterns are based predominantly off studies of men that are not powered to guide management in women. Women are likely to derive similar benefit compared to men without adverse effects for medications such as statins, ezetimibe, and other lipid-lowering therapies.5,6 Yet, other data examining women with heart failure with reduced ejection fraction (HFrEF) suggest that women may derive therapeutic benefit of beta-blockers, ACE-inhibitors, and angiotensin receptor blockers at lower doses than men.7 Such variability in results further highlights a need for balanced representation and analysis.
The Roadblocks
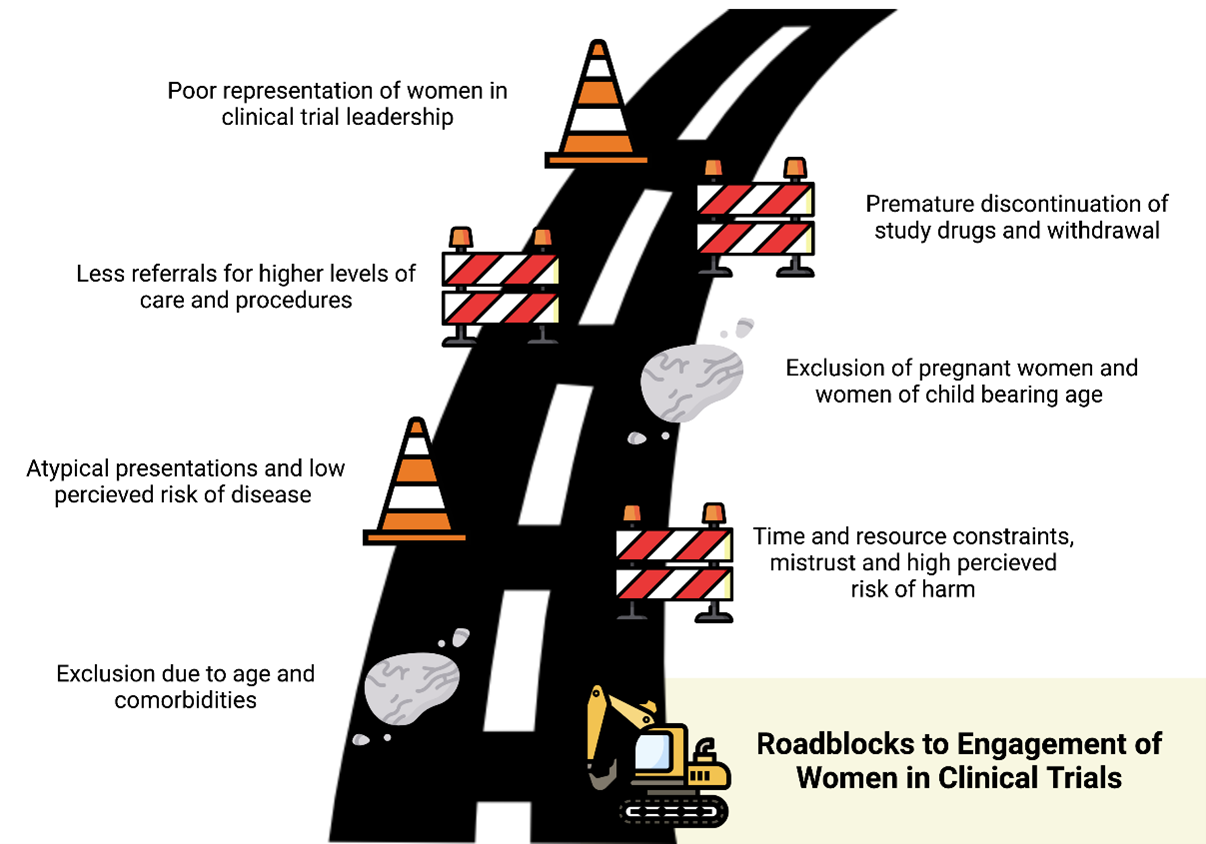
A recent review by Cho et al. highlights the challenges and barriers to enrolling women in cardiovascular trials ranging from disease presentation to individual biases as well as limitations to structural societal beliefs and differential practicing patterns.8 These barriers are shown in Figure 1. This review also details specific recommendations for addressing the barriers.
Figure 1
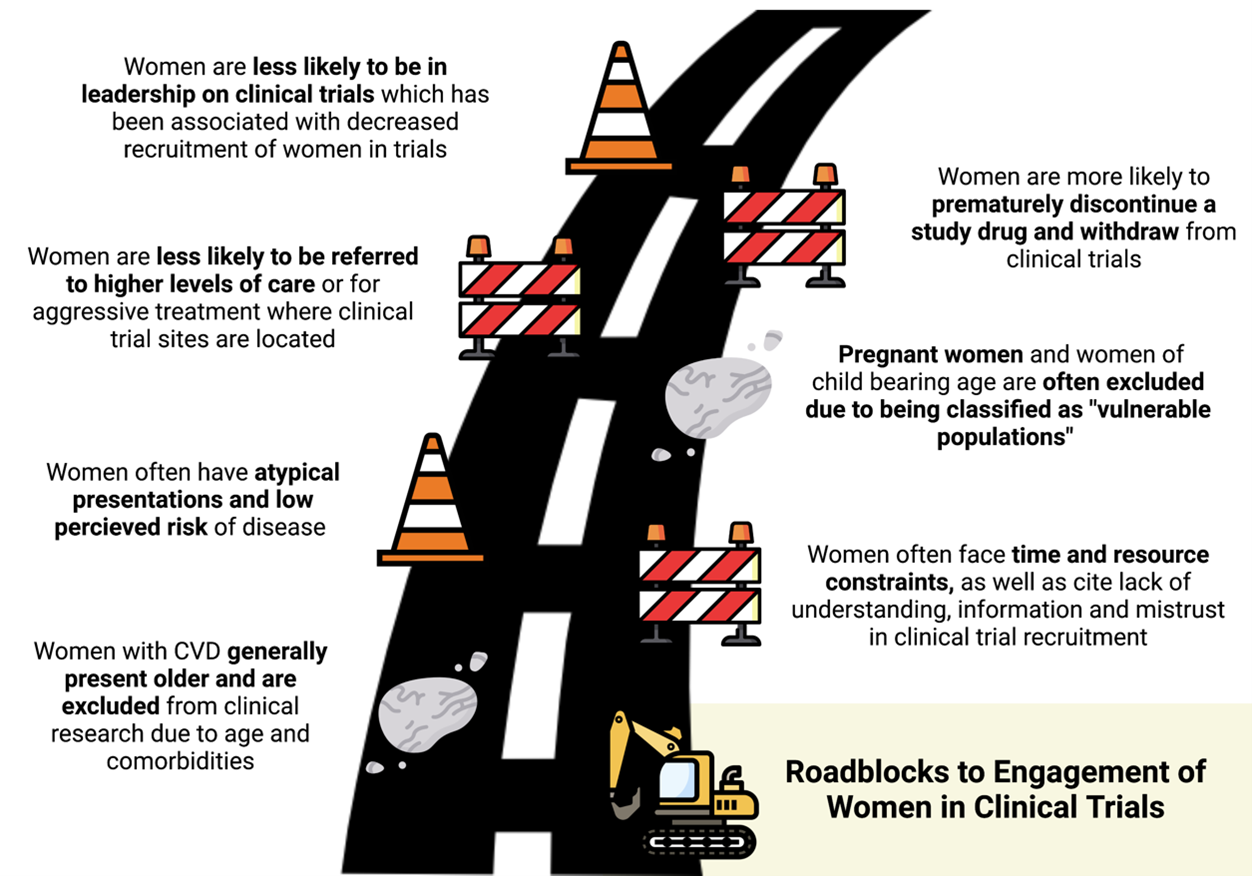
Disease Specific Presentations: Women often present with CVD with multiple comorbidities at an older age.9 Unfortunately, many trials exclude older patients in their recruitment, thus limiting the inclusion of many women who typically present about 1 decade later than men on average and may have decreased functional status from other comorbidities.10 Women are also thought to have atypical disease presentations often leading to lower perceived risk of disease and undertreatment with appropriate medical therapies.11,12
Women are more likely to present with ACS with non-specific symptoms and myocardial infarction with non-obstructed coronary arteries (MINOCA).13 Similarly, women with HF are more likely than men to have heart failure with preserved ejection fraction (HFpEF) rather than reduced ejection fraction. Cho et al. note that despite these recognized differences in presentations, there is limited research into the underlying mechanisms and under-representation of women in the dedicated trials.8
Individual Concerns: Under-represented minority women often cite time and resource constraints, overall lack of understanding, higher perceived risk of harm, and mistrust as barriers to enrollment in clinical trials.14,15 Addressing gaps in outreach and awareness, while also expanding hours and locations for clinical trials sites, could mitigate these obstacles.8,15 Once women are successfully enrolled in a trial, they are more likely to prematurely discontinue the study drug and withdraw from the study.16 Previous trials were not structured to identify the cause of discontinuation; however, further analysis and focus on this topic are critical to identify underlying barriers for study retention.
Clinical Practice Patterns: Historically, women with CVD are less likely than men to be referred to higher levels of care including subspecialty clinics or tertiary care centers where trial enrollments often take place.17 Women are also less likely to receive aggressive treatment and procedures including coronary angiography or ablations, thus further limiting inclusion in ACS, CAD, and atrial fibrillation trials.18 Use of multidisciplinary strategies to improve awareness of ongoing trials and/or increasing trial sites in more under-represented locations can mitigate the logistical barriers to enrollment.8
Structural Changes: Reza et al. published results showing that increased representation of women authorship was directly associated with increased enrollment of women authorship in clinical trials.19 Cho et al. highlight that women representation is often lacking at leadership or principal investigator of trial design, and leadership and increasing leadership diversity could also promote enrollment and higher quality research.8
Special Populations: Pregnant women and women of child-bearing potential are often excluded from trials due to their classification as a "vulnerable population".20 A growing number of pregnant and child-bearing women are developing CVD and are at high risk of complications from pre-eclampsia, gestational hypertension, and/or cardiomyopathy.21 However, in excluding pregnant women from these trials, there is limited data to guide management and target therapies for women with CVD. Cho et al. highlights the need to protect mother and fetus and recommends drug studies on pregnant animals prior to inclusion of women in clinical trials.8
Figure 2
Conclusions
Barriers to enrollment, retention, and equal representation of women in clinical research occurs at every level of trial development. Addressing these barriers not only requires careful attention to individual bias but also an overhaul of current systemic practices. These changes are crucial to allow us to provide optimal evidence-driven care for the growing number of women with CVD globally.
References
- Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139-e596.
- Khan SU, Bashir ZS, Khan MZ, et al. Trends in cardiovascular deaths among young adults in the United States, 1999 to 2018. Am J Cardiol 2020;128:216–17.
- Poon R, Khanijow K, Umarjee S, et al. Participation of women and sex analyses in late-phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007-2009. J Womens Health 2013;22:604-16.
- Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women's participation in cardiovascular clinical trials from 2010 to 2017. Circulation 2020:540–48.
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010;121:1069–77.
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012;59:572–82.
- Santema BT, Ouwerkerk W, Tromp J, et al. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet 2019;394:1254–63.
- Cho L, Vest AR, O'Donoghue ML, et al. Increasing participation of women in cardiovascular trials: JACC Council Perspectives. J Am Coll Cardiol 2021;78:737-51.
- Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med 1999;341:226–32.
- Nanna MG, Chen ST, Nelson AJ, Navar AM, Peterson ED. Representation of older adults in cardiovascular disease trials since the inclusion across the lifespan policy. JAMA Intern Med 2020;180:1531–33.
- Liu J, Saeed A, Hussain A, Virani SS. The way to a woman's heart: assessing, personalizing, and reclassifying atherosclerotic cardiovascular disease risk in female patients. Texas Heart Inst J 2020;47:125–27.
- Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation 2005;111:499–510.
- Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:E891–E908.
- Clar Lt, Watkins L, Pina IL, et al. Increasing diversity in cinical trials: overcoming critical barriers. Curr Probl Cardiol 2019;44:148–72.
- van Diemen J, Verdonk P, Chieffo A, et al. The importance of achieving sex- and gender-based equity in clinical trials: a call to action. Eur Heart J 2021;42:2990–94.
- Lau ES, Braunwald E, Morrow DA, et al. Sex, permanent drug discontinuation, and study retention in clinical trials : Insights From the TIMI trials. Circulation 2021;143:685–95.
- Cook NL, Ayanian JZ, Orav EJ, Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation 2009;119:2463–70.
- Kaiser DW, Fan J, Schmitt S, et al. Gender differences in clinical outcomes after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2016;2:703-10.
- Reza N, Tahhan AS, Mahmud N, et al. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circ Heart Fail 2020:261–270.
- Liu KA, Mager NAD. Women's involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada) 2016;14:708.
- American College of Obstetricians and Gynecologists' Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 212: pregnancy and heart disease. Obstet Gynecol 2019;133:E320–E356.
Clinical Topics: Acute Coronary Syndromes, Arrhythmias and Clinical EP, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Atherosclerotic Disease (CAD/PAD), Atrial Fibrillation/Supraventricular Arrhythmias, Lipid Metabolism, Nonstatins, Novel Agents, Statins, Acute Heart Failure, Interventions and ACS, Interventions and Coronary Artery Disease, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging, Hypertension
Keywords: Heart Failure, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Cost of Illness, Angiotensin Receptor Antagonists, Cardiovascular Diseases, Angiotensin-Converting Enzyme Inhibitors, Coronary Angiography, Coronary Artery Disease, Hypertension, Pregnancy-Induced, Acute Coronary Syndrome, Ezetimibe, Atrial Fibrillation, Prevalence, Pre-Eclampsia, Benchmarking, Pregnant Women, Vulnerable Populations, Practice Patterns, Physicians', Tertiary Care Centers, Leadership, Cause of Death, Functional Status, Pulmonary Artery, Stroke Volume, Myocardial Infarction, Cardiomyopathies, Selection Bias, Lipids, Pharmaceutical Preparations, Female, Clinical Trial
< Back to Listings