Management of Pericarditis in Rheumatic Diseases
Quick Takes
- Pericarditis can arise in systemic rheumatic conditions, particularly in connective tissue diseases such as systemic lupus erythematosus.
- Management of pericarditis associated with rheumatic diseases depends on the specific rheumatic disease diagnosis, with careful consideration of comorbidities and concomitant immunosuppressive agents.
Introduction
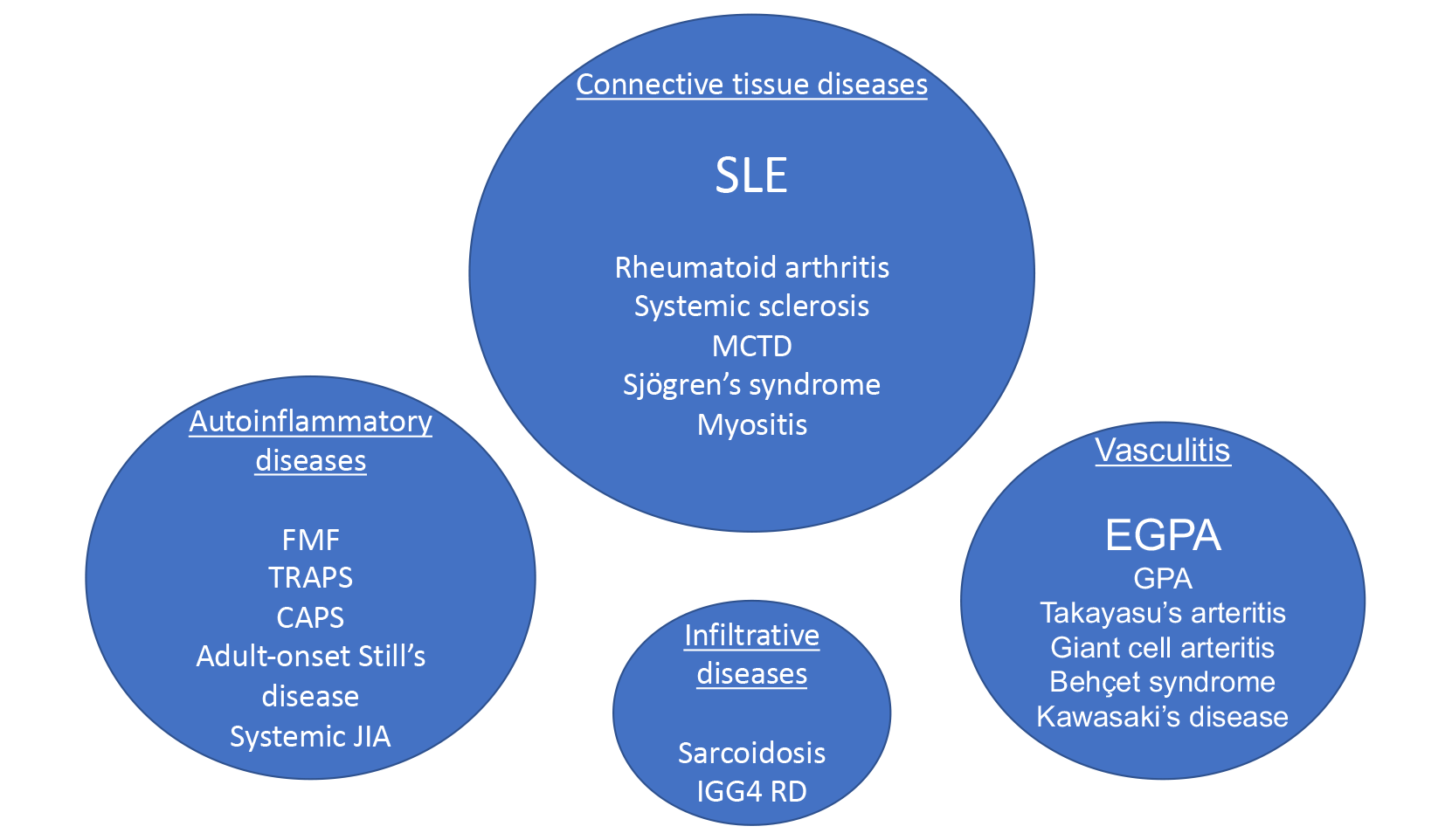
Pericarditis can occur as a manifestation of systemic rheumatic diseases. Presentations range from asymptomatic pericardial effusion, acute and recurrent pericarditis, cardiac tamponade, and rarely, constrictive pericarditis. The prevalence of pericardial disease depends on the specific autoimmune disease with the highest prevalence among those with systemic lupus erythematosus (SLE). Pericarditis can also be seen in other connective tissue diseases including rheumatoid arthritis, Sjögren's syndrome, and systemic sclerosis, and in systemic vasculitis, sarcoidosis, and autoinflammatory syndromes (Figure 1).1,2
Figure 1: Systemic Autoimmune and Autoinflammatory Diseases Associated With Pericarditis
Pericardial effusions may be noted incidentally on echocardiograms or imaging performed for other indications in patients with known rheumatic diseases. In other cases, pericarditis may be the initial presentation of an autoimmune or autoinflammatory disease. Therefore, a detailed history, review of systems, and physical exam should be performed to evaluate for rheumatic disease in patients with pericarditis without a clear underlying cause (Table 1). Laboratory evaluation should be guided by the initial history and exam. Of note, antinuclear antibody (ANA) testing is frequently ordered in clinical practice, but low-level ANA titers (1:40–1:80) can be seen in patients with idiopathic recurrent pericarditis without any systemic autoimmune disease.3 Rheumatology consultation is recommended when an underlying rheumatic disease is suspected in a patient with pericarditis.
Table 1: Select History, Review of Systems, and Initial Laboratory Tests Associated With Rheumatic Diseases*
| Review of systems: | |
| Constitutional: Fevers, fatigue, night sweats | |
| HEENT: Headache, red/painful eyes, vision loss, jaw claudication, sinus congestion, nasal crusting, dry eyes/dry mouth | |
| Cardiac: Dyspnea on exertion, chest pain, palpitations | |
| Pulmonary: Dyspnea, cough, hemoptysis | |
| GI: dysphagia, nausea, abdominal pain, diarrhea | |
| Renal: Foamy or dark urine | |
| Musculoskeletal: Joint pain and swelling, morning stiffness, puffy fingers | |
| Mucocutaneous: Rash, alopecia, oral or nasal ulcers, photosensitivity, skin tightening | |
| Vascular: Raynaud's phenomenon, limb claudication | |
| Neurologic: Numbness/tingling, proximal muscle weakness, seizures, psychosis | |
| Relevant history: | |
| History of thromboembolic disease | |
| History of recurrent miscarriages | |
| History of adult-onset asthma, frequent sinus infections or polyps | |
| History of interstitial lung disease or pulmonary hypertension | |
| Family history of autoimmune diseases | |
| History of illicit drug use and prescription drugs associated with drug-induced SLE | |
| Select laboratory tests: | Notes: |
| Complete blood count with differential | Look for leukopenia, anemia, thrombocytopenia, and eosinophilia |
| Chemistry and liver function tests | Renal involvement important to evaluate in SLE, Sjögren's, systemic sclerosis, ANCA vasculitis |
| Urinalysis with sediment, protein/creatinine ratio, microalbumin/creatinine ratio | Look for active sediment, proteinuria |
| Erythrocyte sedimentation rate and C-reactive protein | Nonspecific markers of inflammation but often elevated in active autoimmune and autoinflammatory diseases |
| Antinuclear antibody | Titer ≥1:80 is considered positive; seen in SLE and other CTD, Hashimoto's thyroiditis, non-rheumatic diseases |
| Smith | More specific for SLE |
| Double-stranded DNA | More specific for SLE, associated with lupus nephritis and may track with disease activity |
| U1RNP | Associated with MCTD, SLE |
| Antiphospholipid antibodies | Includes lupus anticoagulant, Beta-2 glycoprotein I, cardiolipin and phosphatidylserine antibodies |
| Ro (SSA), La (SSB) | Seen in Sjögren's syndrome and many other CTD including SLE, RA, MCTD; associated with neonatal lupus and congenital heart block |
| Rheumatoid Factor | Found in RA, Sjögren's, other CTD, infections and non-rheumatic diseases |
| Anti-cyclic citrullinated peptide (CCP) | More specific for RA than rheumatoid factor |
| Scl-70, RNA polymerase III | Diffuse systemic sclerosis; Scl-70 is associated with increased risk of ILD, RNA polymerase III with risk of scleroderma renal crisis |
| Centromere antibodies or ANA in centromere pattern | Limited systemic sclerosis; association with pulmonary arterial hypertension |
| Jo-1, other myositis-specific antibodies | Inflammatory myositis and anti-synthetase syndrome |
| ANCA, myeloperoxidase and proteinase 3 antibodies | ANCA-associated vasculitis; EGPA patients can be ANCA negative |
*Not designed to be a comprehensive list of symptoms or laboratory tests
Management
Management of incidental pericardial effusion or minimally symptomatic pericarditis in rheumatic disease patients should be guided by usual treatment guidelines for the specific underlying diagnosis. For symptomatic pericarditis, first-line agents include non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine as in idiopathic pericarditis.2 For second-line treatment, low or moderate-dose steroids (0.2-0.5mg/kg) with slow taper are typically used in idiopathic pericarditis,4 but higher doses including 1mg/kg of prednisone (or equivalent) and occasionally pulse intravenous methylprednisolone may be necessary to treat active SLE or vasculitis flares. In such cases, the steroid dose and taper should be determined in collaboration with a rheumatologist. Pericarditis flares while tapering steroids may necessitate the use of steroid-sparing agents such as IL-1 blockers.4 When starting immunosuppressive agents, we recommend checking hepatitis B and C serologies, screening for latent tuberculosis, and ensuring that patients are vaccinated against vaccine-preventable conditions prior to initiation whenever possible. Caution should be exercised when combining IL-1 inhibitors with other immunosuppressive therapies due to the increased risk of infections.5 Intravenous immunoglobulins have been used to treat refractory pericarditis in small studies,6 and may be considered when there are other indications for use, concerns about immunosuppression, or in refractory cases. See Table 2 for a summary of medications and safety considerations. Select disease-specific treatment recommendations are below.
Table 2: Medications Used For Treatment of Pericarditis and Rheumatic Disease-Specific Considerations
| Medication | Dosing | Typical use in rheumatic diseases | Special considerations |
| NSAIDs | Varies by agent | Inflammatory arthritis | Caution in patients with renal disease or history of GI bleeding |
| Colchicine | 0.6mg daily or twice po daily | Autoinflammatory syndromes, gout | Caution in patients with renal disease; use with caution in patients with cytopenia due to risk of myelosuppression; risk of myotoxicity; GI side effects, frequent interactions with other medications |
| Prednisone | Initially 0.2-0.5 mg/kg; up to 1mg/kg po daily or pulse IV methylprednisolone in severe rheumatic diseases | Many autoimmune diseases including SLE, RA, vasculitis, sarcoidosis | Consider pneumocystis prophylaxis if on ≥20mg for more than 4 weeks; consider calcium and vitamin D supplementation and osteoporosis screening, consider GI protection |
| Anakinra | 100mg subcutaneously once daily | FDA-approved for RA, CAPS, gout | Fast-acting; require daily dosing; injection-side reactions; if CrCl <30, consider every other day dosing |
| Rilonacept | 320mg loading dose followed by 160mg subcutaneously once weekly | FDA-approved for CAPS, recurrent idiopathic pericarditis | No data yet on use in pericarditis in patients with an underlying rheumatic condition |
| Canakinumab | 2mg-4mg/kg subcutaneously every 4 weeks | FDA-approved for CAPS, TRAPS, FMF, HIDS, Adult-onset Still's disease, systemic JIA | Limited data in pericarditis; dosing every 4 weeks |
| Azathioprine | Start at 50mg daily and titrate up to 1-2mg/kg po daily | SLE, vasculitis, myositis, RA | Check for TPMT deficiency prior to initiation and avoid if deficient; monitor for leukopenia, LFT abnormalities |
| Mycophenolate mofetil | Start at 500mg po twice daily, titrate to 1000-1500mg po twice daily | SLE, MCTD, systemic sclerosis, myositis, non-severe vasculitis | Monitor for bone marrow suppression, LFT abnormalities; GI side effects common; avoid in pregnancy and caution women of childbearing age |
| IVIG | 2g/kg IV divided over 3-5 days | Kawasaki's disease, myositis, and immune thrombocytopenia | Caution in those with history of heart failure and thromboembolism |
Systemic Lupus Erythematosus
SLE is a systemic autoimmune disease characterized by autoantibody formation and immune complex deposition. More than 50% of SLE patients have some pericardial involvement, though a majority have asymptomatic/incidental pericardial effusions.7 However, acute pericarditis, and rarely, cardiac tamponade can occur.8
Choice of treatment for SLE pericarditis often depends on other organ involvement and comorbidities. NSAIDs can be used in SLE patients with mild pericarditis who do not have significant renal disease. Colchicine has been used effectively in SLE pericarditis as a steroid-sparing drug; prolonged treatment beyond 3 months may be necessary due to the elevated risk of recurrence in this population.9 Steroids are used to treat other organ manifestations of SLE and can be used in SLE pericarditis but have significant short and long-term adverse effects. Therefore, steroids should be used at the lowest effective dose for the shortest duration, and steroid-sparing agents should be considered. In severe cases of SLE pericarditis with tamponade physiology, pericardiocentesis or pericardial window may be necessary.
Other immunosuppressive agents used to treat SLE include hydroxychloroquine, azathioprine, mycophenolate mofetil (MMF), methotrexate, calcineurin inhibitors, cyclophosphamide and belimumab; choice of agent is based on disease severity and specific organ manifestations.10 Of these, azathioprine has the most robust data on use in recurrent pericarditis4,11 and should be considered as a steroid-sparing agent in refractory or recurrent SLE pericarditis and in pregnant SLE patients with pericarditis. Data is limited on other steroid-sparing agents, but expert consensus also supports the use of MMF for treatment of SLE pericarditis.12 Recently, IL-1 inhibition has emerged as a highly effective therapy for recurrent pericarditis.13 Data on IL-1 inhibition in SLE is limited so far, with case reports using anakinra to treat refractory pericarditis in patients with SLE who failed other treatments.14 Anakinra and rilonacept can be considered as steroid-sparing agents in cases of refractory or recurrent SLE pericarditis for whom NSAIDs and colchicine are ineffective or contraindicated.
Vasculitis
Vasculitis includes several autoimmune conditions in which inflammatory cells infiltrate blood vessel walls, resulting in ischemia, thrombosis, end-organ damage, or aneurysm formation. Patients often experience constitutional symptoms and have elevated inflammatory markers and evidence of other end-organ dysfunction.15 Of the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides, pericarditis was most frequently observed in eosinophilic granulomatosis with polyangiitis at 14.4%, compared to 4.3% in granulomatosis with polyangiitis and 5.2% in microscopic polyangiitis.16 A majority of the ANCA vasculitis patients with pericarditis were considered to have severe vasculitis and treated accordingly with high dose steroids plus rituximab or cyclophosphamide.17 With the exception of Kawasaki disease, pericarditis has rarely been reported in medium or large vessel vasculitis.
Autoinflammatory Syndromes
Periodic fever syndromes such as familial Mediterranean fever, tumor necrosis factor receptor-associated periodic fever syndrome, and cryopyrin-associated periodic syndromes are familial conditions arising from specific genetic mutations that lead to aberrant innate immune system activation.18 Patients may present with recurrent episodes of fevers, serositis, rash, aphthous ulcers, gastrointestinal symptoms, and arthralgias; pericarditis has rarely been reported during flares. Pericarditis has also been reported in systemic juvenile idiopathic arthritis and adult-onset Still's disease, which are sometimes considered polygenic autoinflammatory syndromes.19,20 Treatments for these conditions overlap substantially with treatments for pericarditis: NSAIDs, colchicine, steroids, and IL-1 blockers.
Conclusion
Autoimmune and autoinflammatory conditions can present with a spectrum of pericardial disease. Clinicians should maintain a high index of suspicion for rheumatic diseases in patients with pericarditis who have an inflammatory profile or evidence of end-organ dysfunction. Treatment of autoimmune pericarditis should be guided by the specific rheumatologic diagnosis, other organ involvement, and other immunosuppressive medications that are being used to treat the underlying disease. Collaboration with a rheumatologist may be beneficial.
References
- Prasad M, Hermann J, Gabriel SE, et al. Cardiorheumatology: cardiac involvement in systemic rheumatic disease. Nat Rev Cardiol 2015;12:168–76.
- Bizzi E, Trotta L, Pancrazi M, et al. Autoimmune and autoinflammatory pericarditis: definitions and new treatments. Curr Cardiol Rep 2021;23:128.
- Imazio M, Brucato A, Doria A, et al. Antinuclear antibodies in recurrent idiopathic pericarditis: prevalence and clinical significance. Int J Cardiol 2009;136:289-93.
- Imazio M, Lazaros G, Brucato A, Gaita F. Recurrent pericarditis: new and emerging therapeutic options. Nat Rev Cardiol 2016;13:99-105.
- Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004;50:1412-19.
- Imazio, M, Lazaros, G, Picardi, E, et al. Intravenous human immunoglobulins for refractory recurrent pericarditis: a systematic review of all published cases. J Cardiovas Med 2016;17:263-69.
- Zagelbaum Ward NK, Linares-Koloffon C, Posligua A, et al. Cardiac manifestations of systemic lupus erythematous: an overview of the incidence, risk factors, diagnostic criteria, pathophysiology and treatment options. Cardiol Rev 2022;30:38-43.
- Goswami RP, Sircar G, Ghosh A, Ghosh P. Cardiac tamponade in systemic lupus erythematosus. QJM 2018;111:83-87.
- Morel N, Bonjour M, Le Guern V, et al. Colchicine: a simple and effective treatment for pericarditis in systemic lupus erythematosus? A report of 10 cases. Lupus 2015;24:1479–85.
- Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis 2021;80:14–25.
- Vianello F, Cinetto F, Cavraro M, et al. Azathioprine in isolated recurrent pericarditis: a single centre experience. Int J Cardiol 2011;147:477-8.
- Muangchan C, van Vollenhoven RF, Bernatsky SR, et al. Treatment algorithms in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015;67:1237-45.
- Klein, AL, Imazio M, Cremer P, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 2021:384:31-41.
- Cafarelli F, Coladonato L, Lopalco G, Cacciapaglia F, Cantarini L, Iannone F. Successful treatment with anakinra of refractory pericarditis in systemic lupus erythematosus. Clin Exp Rheumatol 2021;39:227.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1-11.
- Thompson GE, Bourne MH Jr., Moura MC, et al. Pleuritis and pericarditis in antineutrophil cytoplasmic autoantibody-associated vasculitis. Chest 2021;160:572-81.
- Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken) 2021;73:1088-1105.
- Nigrovic PA, Lee PY, Hoffman HM. Monogenic autoinflammatory disorders: conceptual overview, phenotype, and clinical approach. J Allergy Clin Immunol 2020;146:925-37.
- Bodard Q, Langlois V, Guilpain P, et al. Cardiac involvement in adult-onset Still's disease: manifestations, treatments, and outcomes in a retrospective study of 28 patients. J Autoimmun 2021;116:102541.
- Di Cola I, Di Muzio C, Conforti A, et al. Adult-onset Still's disease with elderly onset: results from a multicentre study. Clin Exp Rheumatol 2022;40:1517-25.
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Pericardial Disease, Vascular Medicine, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, Congenital Heart Disease, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Lipid Metabolism, Novel Agents, Statins, Heart Failure and Cardiac Biomarkers, Interventions and Vascular Medicine
Keywords: Aneurysm, Antibodies, Antineutrophil Cytoplasmic, Antibodies, Antinuclear, Antigen-Antibody Complex, Anti-Inflammatory Agents, Anti-Inflammatory Agents, Non-Steroidal, Arthralgia, Arthritis, Juvenile, Arthritis, Rheumatoid, Autoimmune Diseases, Azathioprine, Calcineurin Inhibitors, Cardiac Tamponade, Churg-Strauss Syndrome, Colchicine, Connective Tissue Diseases, Consensus, Cryopyrin-Associated Periodic Syndromes, Cyclophosphamide, Exanthema, Familial Mediterranean Fever, Fever, Granulomatosis with Polyangiitis, Hepatitis B, Hereditary Autoinflammatory Diseases, Hydroxychloroquine, Immune System, Immunoglobulins, Intravenous, Immunosuppressive Agents, Interleukin 1 Receptor Antagonist Protein, Interleukin-1, Ischemia, Latent Tuberculosis, Lupus Erythematosus, Systemic, Methotrexate, Methylprednisolone, Microscopic Polyangiitis, Mucocutaneous Lymph Node Syndrome, Multiple Organ Failure, Mutation, Mycophenolic Acid, Pericardial Effusion, Pericardiocentesis, Pericarditis, Pericarditis, Constrictive, Physical Examination, Prednisone, Pregnancy, Prevalence, Receptors, Tumor Necrosis Factor, Referral and Consultation, Rheumatic Diseases, Rheumatologists, Rheumatology, Rituximab, Sarcoidosis, Scleroderma, Systemic, Serositis, Severity of Illness Index, Steroids, Stomatitis, Aphthous, Syndrome, Systemic Vasculitis, Thrombosis, Vaccines, Vasculitis
< Back to Listings