The OPTION Trial: Percutaneous Left Atrial Appendage Occlusion Devices Rise as an Option Post Ablation
Quick Takes
- The OPTION (Comparison of Anticoagulation With Left Atrial Appendage Closure After Atrial Fibrillation Ablation) trial was a prospective, randomized, multicenter trial comparing percutaneous left atrial appendage occlusion (LAAO) with oral anticoagulation (OAC) in >1,500 patients with elevated stroke risk after catheter ablation for the treatment of atrial fibrillation (AF).
- The OPTION trial results demonstrated that LAAO was noninferior to OAC for the composite primary endpoint of all-cause death, stroke, or systemic embolization and was superior to OAC for non–procedure-related bleeding.
- Percutaneous LAAO with the WATCHMAN FLX (Boston Scientific) may be an alternative to OAC in patients with elevated stroke risk after catheter ablation to treat AF.
Atrial Fibrillation Ablation
Catheter ablation has become an effective treatment strategy for patients with AF. In 2009, the ThermoCool AF (NAVISTAR® THERMOCOOL® Catheter for the Radiofrequency Ablation of Symptomatic Paroxysmal Atrial Fibrillation) trial results showed that ablation to treat drug-refractory atrial fibrillation (AF) provided a higher quality of life and lower symptom frequency than did antiarrhythmic drug (AAD) therapy.1 The MANTRA-PAF (Medical Antiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation) study results showed that catheter ablation as first-line therapy was associated with lower AF burden at 24-month follow-up.2 Subsequently, the EARLY-AF (Early Aggressive Invasive Intervention for Atrial Fibrillation) trial results showed that ablation was associated with a lower rate of recurrent atrial arrhythmia at 1-year compared with AAD therapy.3 The CABANA (Catheter ABlation vs ANtiarrhythmic Drug Therapy in Atrial Fibrillation) trial results showed that ablation to treat symptomatic AF did not have a significant difference in the composite of death, stroke, bleeding, or cardiac arrest compared with AAD therapy.4 The STOP AF First (Cryoballoon Catheter Ablation in Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation) trial results showed that cryoballoon catheter ablation was also associated with lower rates of recurrence compared with AAD therapy.5 The FIRE AND ICE trial evaluated radiofrequency ablation versus cryoballoon ablation, and its data showed that cryoballoon ablation was noninferior and had similar safety profiles.6 With the arrival of pulsed field ablation (PFA), the AdmIRE (Assessment of Safety and Effectiveness in Treatment Management of Atrial Fibrillation With the Biosense-Webster Irreversible Electroporation Ablation System) trial results showed that PFA was a safe treatment, with 75.4% freedom from atrial arrhythmia.7 Data from additional clinical trials with PFA showed similar findings. The growing evidence has bumped catheter ablation to a Class 1 recommendation as first-line therapy for specific patient populations (Figure 1).8
Figure 1: Trials Providing Evidence Leading Up to the OPTION Trial
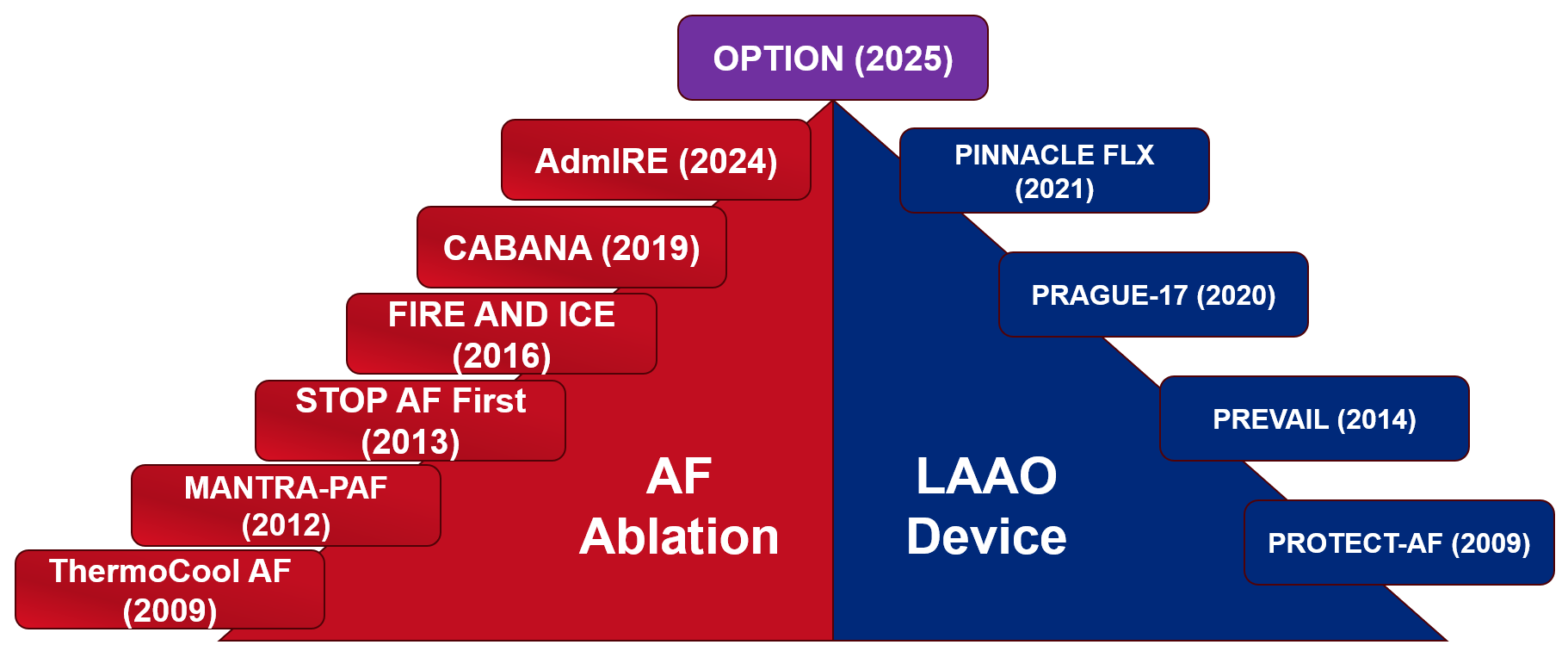
AdmIRE = Assessment of Safety and Effectiveness in Treatment Management of Atrial Fibrillation With the Biosense-Webster Irreversible Electroporation Ablation System; AF = atrial fibrillation; CABANA = Catheter ABlation vs ANtiarrhythmic Drug Therapy in Atrial Fibrillation; LAAO = left atrial appendage occlusion; MANTRA-PAF = Medical Antiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation; OPTION = Comparison of Anticoagulation With Left Atrial Appendage Closure After Atrial Fibrillation Ablation; PINNACLE FLX = Protection Against Embolism for Nonvalvular AF Patients: Investigational Device Evaluation of the Watchman FLX LAA Closure Technology; PRAGUE-17 = Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in High-Risk Atrial Fibrillation Patients; PREVAIL = Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy; PROTECT AF = WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation; STOP AF First = Cryoballoon Catheter Ablation in Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation; ThermoCool AF = NAVISTAR® THERMOCOOL® Catheter for the Radiofrequency Ablation of Symptomatic Paroxysmal Atrial Fibrillation.
The 2023 multisociety Guideline for the Diagnosis and Management of AF recommends that oral anticoagulation (OAC) be continued for ≥3 months after the procedure and that long-term OAC should be dictated by a patient's stroke risk.8 Therefore, those at high risk (i.e., CHA2DS2-VASc score ≥2 in men and ≥3 in women) would need to continue with OAC.
Left Atrial Appendage Occlusion Devices
In recent years, left atrial appendage occlusion (LAAO) devices have emerged as an option for stroke prevention as an alternative to OAC. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) trial results showed that percutaneous LAAO was noninferior to warfarin regarding both bleeding and thromboembolic prevention.9 The PREVAIL (Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) trial results showed that LAAO did not achieve noninferiority for embolic events, but the study was limited by lower overall event rates.10 The PRAGUE-17 (Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in High-Risk Atrial Fibrillation Patients) trial results showed that, in patients at high risk of stroke and increased bleeding risk, LAAO was noninferior to direct OAC for prevention of AF-related complications.11 The PINNACLE FLX (Protection Against Embolism for Nonvalvular AF Patients: Investigational Device Evaluation of the Watchman FLX LAA Closure Technology) study evaluated a newer-generation LAAO device that demonstrated high procedural success and low thromboembolic event rates.12 These studies all involved patients who were at high bleeding risk, which leads to the question of whether LAAO is an option for patients without high bleeding risk undergoing AF ablation.
The OPTION Trial
The OPTION (Comparison of Anticoagulation With Left Atrial Appendage Closure After Atrial Fibrillation Ablation) trial examined, in a prospective, randomized, multicenter trial design, whether percutaneous LAAO was noninferior to OAC regarding the composite endpoint of death, stroke, or systemic thromboembolism at 36 months following catheter ablation in patients with nonvalvular atrial fibrillation (NVAF) at high stroke risk (i.e., CHA2DS2-VASc score ≥2 in men and ≥3 in women). The primary safety endpoint was superiority of LAAO to OAC in major or clinically relevant bleeding at 36 months.13
Patients were recruited between Nov. 6, 2019, and June 28, 2021, from 106 sites in 10 countries. They could either have catheter ablation within 90-180 days before randomization or catheter ablation could be performed within 10 days of randomization. Patients underwent 1:1 randomization to ablation followed by OAC versus ablation with LAAO. Block randomization was done to further stratify on the basis of a sequential LAAO procedure (ablation 90-180 days prior to randomization) versus a concomitant LAAO procedure (ablation within 10 days of randomization).13
The inclusion criteria included patients with prior ablation of NVAF between 90 and 180 days prior to randomization or ablation within 10 days of randomization with an elevated stroke risk (CHA2DS2-VASc score ≥2 in men and ≥3 in women). Excluded patients included those who required long-term anticoagulation for another reason; who had recent stroke, myocardial infarction, or major bleeding event; who had a history of atrial septal defect/patent foramen ovale device; or who had left ventricular ejection fraction <30%.13
A total of 1,600 patients underwent randomization: 803 underwent ablation and LAAO whereas 797 underwent ablation and OAC. At 36-month follow-up, 758 patients remained in the ablation-and-LAAO group and 737 in the ablation-and-OAC group.13
Does OPTION Provide Enough Evidence for an LAAO After or Combined With Ablation?
The primary endpoint (a composite of all-cause death, stroke, or systemic embolism) occurred in 5.3% of patients in the LAAO-and-ablation group and in 5.8% in the ablation-and-OAC group (pnoninferiority < 0.001). The primary safety endpoint of non–procedure-related bleeding occurred in 8.5% of patients in the LAAO-and-ablation group and in 18.1% in the OAC-and-ablation group (psuperiority < 0.001).13
The secondary endpoint of major bleeding events occurred in 3.9% of patients in the LAAO-and-ablation group and in 5% in the OAC-and-ablation group (p < 0.001) (Table 1).13
Table 1: Primary and Secondary Outcomes in the OPTION Trial
|
Endpoint
|
Analysis
|
No. of Pts in Device Group (%) a
|
No. of Pts in Anticoagulation Group (%) b
|
Difference (One-Sided 97.5% Upper Confidence Limit)
|
|
Primary Endpoints c
|
||||
|
Safety
|
Superiority
|
85 (8.5)
|
137 (18.1)
|
–
|
|
Efficacy
|
Noninferiority d
|
41 (5.3)
|
44 (5.8)
|
-0.5 (1.8) percentage points
|
|
Secondary Endpoint c
|
||||
|
Major bleeding event
|
Noninferiority d
|
30 (3.9)
|
38 (5)
|
-1.1 (1) percentage points
|
a n = 803
b n = 797
c p < 0.001 for all endpoints
d With a margin of 5 percentage points
No. = number; OPTION = Comparison of Anticoagulation With Left Atrial Appendage Closure After Atrial Fibrillation Ablation; Pts = patients.
Overall, the OPTION trial data present compelling evidence that percutaneous LAAO may be an alternative to OAC for patients with NVAF and moderate to elevated stroke risk who have undergone catheter ablation. The trial has received some criticism over the noninferiority and superiority design for its efficacy and safety outcomes and for the inclusion of all-cause death in the composite primary efficacy outcome endpoint. Another criticism is the overall lower observed rate of stroke in both the LAAO and OAC groups.
The OPTION trial was a well-executed trial that addressed an important clinical question in contemporary management of patients with NVAF. More data and studies will be needed to confirm these findings with PFA and newer-generation or other LAAO devices as an option for stroke prevention after ablation. Additional questions that may arise for these patients include the approach to anticoagulation and the management of recurrent arrhythmia, including repeat ablation procedures. A shared decision-making discussion that incorporates the latest available evidence can help guide management for each patient for stroke prevention.
References
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333-340. doi:10.1001/jama.2009.2029
- Walfridsson H, Walfridsson U, Nielsen JC, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation: results on health-related quality of life and symptom burden. The MANTRA-PAF trial. Europace. 2015;17(2):215-221. doi:10.1093/europace/euu342
- Andrade JG, Deyell MW, Macle L, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388(2):105-116. doi:10.1056/NEJMoa2212540
- Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. doi:10.1001/jama.2019.0693
- Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. doi:10.1056/NEJMoa2029554
- Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235-2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Calkins H, Mansour M, et al. Pulsed field ablation to treat paroxysmal atrial fibrillation: safety and effectiveness in the AdmIRE pivotal trial. Circulation. 2024;150(15):1174-1186. doi:10.1161/CIRCULATIONAHA.124.070333
- Writing Committee Members, Joglar JA, Chung MK, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2024;83(1):109-279. doi:10.1016/j.jacc.2023.08.017
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988-1998. doi:10.1001/jama.2014.15192
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1-12. doi:10.1016/j.jacc.2014.04.029
- Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75(25):3122-3135. doi:10.1016/j.jacc.2020.04.067
- Kar S, Doshi SK, Sadhu A, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation. 2021;143(18):1754-1762. doi:10.1161/CIRCULATIONAHA.120.050117
- Wazni OM, Saliba WI, Nair DG, et al. Left atrial appendage closure after ablation for atrial fibrillation. N Engl J Med. 2025;392(13):1277-1287. doi:10.1056/NEJMoa2408308
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Electrophysiology, Atrial Appendage