ACC Scientific Statement Addresses Use of QCPA in Clinical Practice
With the U.S. Food and Drug Administration approving a growing number of products that can perform quantitative coronary plaque analysis (QCPA), a new ACC Scientific Statement provides consensus recommendations on the appropriate use of QCPA in clinical practice.
The statement, published in JACC: Cardiovascular Imaging and led by Chair Y.S. Chandrashekhar, MD, FACC, and Vice Chairs Ron Blankstein, MD, FACC, and Leslee J. Shaw, PhD, FACC, was developed by a panel of experts convened by the College to develop guidance for cardiovascular clinicians and imagers. It focuses on clinical indications, methods for interpretation and reporting, standardization, and future research directions.
"Initial published data on QCPA have focused on measures of accuracy, prognostic value, and impact on therapeutic decision making," write Chandrashekhar and colleagues. "However, there is no consensus on when or how to use and interpret QCPA in clinical practice, as well as a paucity of data on the utility of serial plaque analysis."
According to the statement, QCPA should be used with patients who have visual evidence of plaque on coronary computed tomography angiography. The authors note that it may also be useful for further assessing risk and helping to guide the initiation or intensification of preventive therapies.
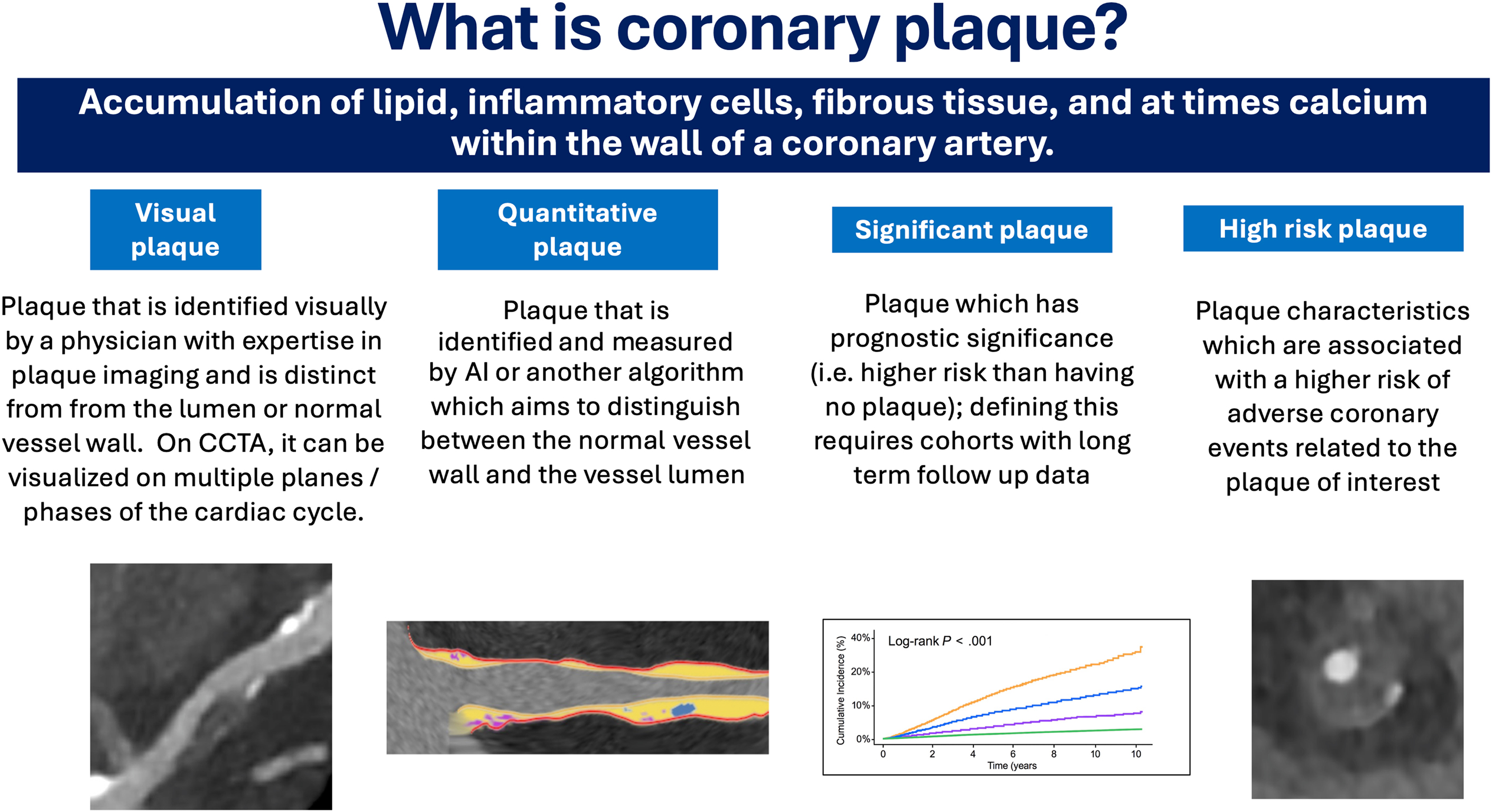
Recommendations on what elements should be included in a physician's QCPA report, as well as a vendor report are also outlined. All physician reports should include quantitative and qualitative QCPA results, total evaluated length of coronary tree, and comparisons to any prior scans as relevant, along with a series of options elements like vessel- and lesion-level information, percentage of atheroma volume, high-risk plaque characteristics, treatment recommendations, etc. Vendor reports should always include the name of vendor/software, image/analysis quality assessment, scanner/kilovoltage peak (kVp), statement on reproducibility data of the QCPA software, quality assurance/quality control performed, and segments excluded from analysis (if any).
On the standardization front, the statement recommends that each QCPA algorithm include published validation against invasive imaging reference data, histology reference data, or both. Additionally, the authors urge each vendor to "set and report a threshold that represents a meaningful amount of total coronary plaque volume based on technical and clinical validation." Ultimately, the statement stresses the need for the creation of a standard data set that is representative of a diverse population and can be used by different vendors for validation and standardization purposes.
Serial coronary QCPA is also addressed, with the statement recommending it "in select clinical scenarios" where information "will result in a meaningful change to patient management." A minimum follow-up interval of at least 2-5 years is recommended for serial QCPA, using the same scan, kVP and reconstruction parameter as the initial scan if possible.
"The Writing Committee recognized that it is important to evaluate changes in plaque composition, and not just [total plaque volume]," according to the statement. "In addition, any changes in QCPA should also be concordant with visual evaluation of the two scans."
Looking ahead, the statement highlights the need for on prognostic, diagnostic, and therapeutic implications of minimal plaque. Currently, "the clinical significance of small amounts of plaque defined by QCPA that is not visualized by the reader (i.e., 20 mm to 30 mm) remains unknown at this time," the authors write.
Keywords: Plaque, Atherosclerotic
< Back to Listings
