Optimizing Outcomes Of Catheter-Based Treatment Of Acute Proximal Deep Venous Thrombosis With Venous Stenting
Quick Takes
- Although restoration of flow in the occluded vein helps in reducing pain, swelling associated with deep vein thrombosis (DVT), the other two long term goals of interventional therapy for DVT (preventing post thrombotic syndrome and recurrent thrombosis) depend upon the adequacy or "completeness" of thrombolysis achieved at the time of initial catheter directed thrombolysis procedure.
- While angiography is the mainstay in arteries, the same is not true for veins. Studies have shown that venography has low sensitivity and may miss significant stenosis or obstruction in the veins. Intravascular ultrasound (IVUS) imaging is critical for adequate assessment of venous stenosis. We suggest that operators should have low threshold for IVUS imaging particularly if they do not plan to perform stenting after catheter directed thrombolysis (CDT) for acute iliofemoral DVT.
Introduction
Interventional catheter-based therapies for acute deep vein thrombosis (DVT) have been rapidly adopted over the recent years1 and have the potential to improve outcomes. Interventional treatments include catheter directed thrombolysis, thrombectomy, venoplasty and venous stenting. In this review, we discuss the utilization and role of venous stenting in patients with acute proximal lower extremity (LE) DVT.
Utilization of Venous Stents in Patients with Acute DVT in Contemporary Practice
There has been an increase in the use of venous stenting as an adjunctive treatment for acute DVT secondary to occlusive or stenotic lesions in the venous outflow. In a surgical series of patients who underwent surgical thrombectomy, the treatment of iliac stent reduced the rethrombosis rates from 72% to 13%.2 In patients undergoing catheter-directed thrombolysis (CDT), the role of stenting has not been very well studied, and in single center studies the stenting rates have varied from 15 to 80%. In an analysis from the nationwide United States (US) sample, we found that out of all the patients who underwent CDT for acute DVT from 2005-2013, (n=7097), only 27% (n=1932) had stent placement.3 During the study period, there was a slow but gradual increase in the rates of venous stenting associated with lower all-cause mortality. The rates of adjunctive stenting during CDT in this nationwide representative sample is like rates of adjunctive stent placement in major randomized controlled trials which vary from 28% in the ATTRACT (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis) trial4 to 45% in the CAVA (CAtheter Versus Anticoagulation Alone for Acute Primary (Ilio)Femoral DVT) trial.5,6 One of the factors which may have led to lower rates of stenting both nationwide and in these randomized trials may be related to low sensitivity of venography to detect significant venous outflow obstruction. None of these randomized trials used routine intravascular ultrasound (IVUS) imaging as a part of the study design and the use of IVUS imaging in the nationwide sample was only 2.7%. In this study those patients that had an IVUS had a stenting rate of 60%, while the stenting rate was only 27% in those who did not get an IVUS. In the CAVA trial those patients with successful CDT had 75% stenting rates as opposed to 11% in those who did not have a stent. The patients that had successful CDT had significantly lower moderate to severe post thrombotic syndrome (PTS) rates.
Importance of "Completeness" of CDT for Acute DVT
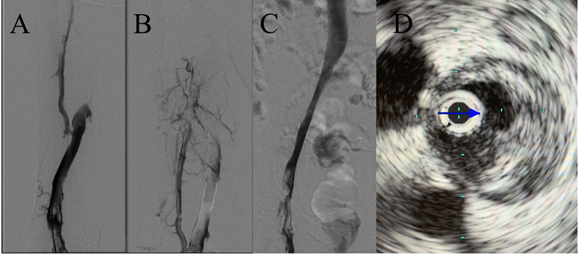
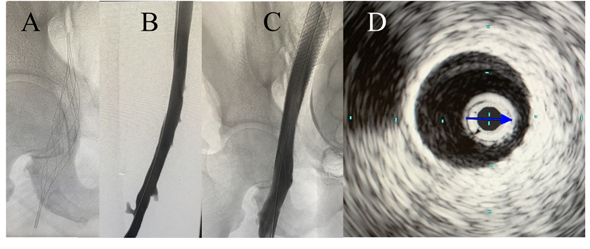
Although restoration of flow in the occluded vein helps in reducing pain and swelling associated with DVT, the other two long term goals of interventional therapy for DVT (preventing post thrombotic syndrome and recurrent thrombosis) depend upon the adequacy or "completeness" of thrombolysis achieved at the time of initial CDT procedure. Adequate thrombus resolution and resolution of venous outflow obstruction or stenosis if present constitute two critical steps in optimizing outcomes for patients with acute DVT (Figures 1 and 2). It is the later aspect that stenting addresses and plays an important role achieving and sustaining a successful revascularization. Venoplasty without stenting may achieve initial luminal gain during CDT, but without stenting is usually not sufficient to prevent recoil of the vein in the long term. The importance of a successful CDT is highlighted by the findings of the CAVA trial, which showed that successful CDT (defined as restoration of ≥90% patency of the vein) was associated with significantly lower rates of moderate to severe post thrombotic syndrome compared to patients who either had unsuccessful CDT or were treated with anticoagulation alone. These studies highlight that a complete or successful CDT followed by stenting to relieve outflow obstruction may be the key to improving long term outcomes of catheter-based thrombus removal in patients with iliofemoral DVT.
Figure 1
Figure 2
Assessment of Venous Obstruction or Stenosis in Acute DVT – Role of IVUS Imaging
While angiography is the mainstay in arteries, the same is not true for veins. Studies have shown that venography has low sensitivity and may miss significant stenosis or obstruction in the veins. A study by Gagne and colleagues compared IVUS imaging with multiplanar venography.7 IVUS imaging identified significant venous lesions in an additional 26% of patients, which were missed by venography. IVUS imaging led to an increase in the use of adjunctive stenting by 23%. This study highlights the crucial role of IVUS imaging as a part of the CDT or pharmacomechanical CDT procedure and the role of this modality in identifying venous outflow obstruction.
Evolution of Venous Stents
Until recently, the Wallstent™ Endoprosthesis Stent (Boston Scientific Corporation) has been the most widely and longest used venous stent. There are several technical challenges associated with the use of this stent. The first issue relates to poor radial strength at the edges, which makes it prone to collapse or external compression. Another issue that relates to the design of this stent is significant foreshortening (up to 40%) during deployment. Due to this issue, precise placement of this may be challenging at times.
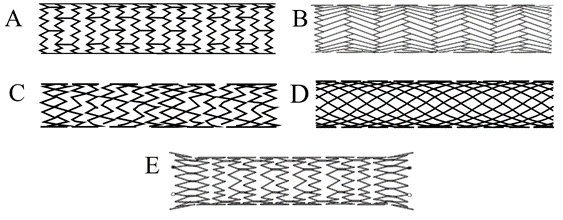
To overcome the challenges related to the use of Wallstent™, newer stents have recently become available (Figure 3). Most of the new stents are based on a Nitinol platform and a non-braided design, which prevents significant foreshortening during deployment. Also, the newer stents can be used with a lower profile delivery system (7French [Fr] for Zilver® Vena™ Venous Self-Expanding Stent, Cook Medical) compared to the 10Fr system required for the Wallstent™. The pivotal trials which tested the safety and efficacy of the Venovo™ Venous Stent (Bard Medical), Zilver® Vena™ (Cook Medical) and the Abre™ (Medtronic Medical) stents also included patients with acute DVT. A comparison of various stents is provided in Table 1.
Figure 3
Table 1: A Comparison of Specifications of Various Stent Platforms
| Fore-shortening during deployment | Sheath size | Studied in acute DVT | 12-month patency rate in pivotal trials | Comments | ||
| Wallstent™ (Boston Scientific Corporation) | Braided design matrix Elgiloy based |
++++ (up to 40%) |
10Fr | Yes | At 4-7 years (90-100%) for non-thrombotic lesions and (74-89%) for thrombotic lesions. For chronic total occlusions (66 - 89%) at 4-7 years. | - Weaker at edges, and prone to collapse due to external compression due to poor radial force - long term use with proven safety track record. |
| Venovo™ Stent (Bard Medical) | Non-braided open cell design Nitinol based |
+ | 8Fr | Yes | 88.3% at 12 months | - Higher frequency of prolonged back pain (especially when oversized) due to Nitinol-based design - Reports of immediate stent underexpansion - Recalled |
| VICI VENOUS STENT® (Boston Scientific Corporation) | Non-braided closed cell design Nitinol based |
++ Less than 20% |
9Fr | No | 84% at 12 months | - Higher frequency of prolonged back pain (especially when oversized) due to Nitinol based design - Reports of stent migration - Recalled |
| Zilver® Vena™ Stent (Cook Medical) | Nitinol based | + | 7Fr | Yes | 89.9% at 12 months | - Higher frequency of prolonged back pain (especially when oversized) due to Nitinol based design |
| Abre™ Stent (Medtronic Medical) | Nitinol based | + | 9Fr | Yes | 79.8% in PTS 87.1% in acute DVT 98.6% in NIVL at 12 months |
- Higher frequency of prolonged back pain (especially when oversized) due to Nitinol based design |
Though pivotal trials have shown promising results and there has been an increase in the use of newer generation stents, there have also been several reports suggesting issues with the newer generation stents (under-expansion of the Venovo™ stents immediately upon deployment,8 stent migration and stent fractures noted with VICI® stent). Both stents have been recalled.9 At this time, these reports appear to be temporary, however more data is needed to better guide the clinicians regarding pitfalls related to these stents.
Conclusion
Revascularization of venous outflow obstruction during CDT is a key component in improving outcomes in patients with acute DVT. Use of IVUS imaging to better assess venous outflow obstruction during CDT followed by stenting to relieve the obstruction has the potential to improve long term clinical outcomes in patients with acute proximal DVT. With improvements in understanding of the pathophysiology and treatment of acute DVT, the availability of newer generation stents that are more user-friendly has broadened the horizon of treatment modalities available for these patients.
References
- Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med 2014;174:1494–1501.
- Hartung O, Benmiloud F, Barthelemy P, Dubuc M, Boufi M, Alimi YS. Late results of surgical venous thrombectomy with iliocaval stenting. J Vasc Surg 2008;47:381–87.
- Tang A, Lakhter V, Zack CJ, et al. Contemporary nationwide trends and in-hospital outcomes of adjunctive stenting in patients undergoing catheter-directed thrombolysis for proximal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2021;9:62-72.e1.
- Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 2017;377:2240–52.
- Notten P, ten Cate-Hoek AJ, Arnoldussen CWKP, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol 2020;7:e40–49.
- Enden T, Haig Y, Kløw NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31–38.
- Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678–87.
- VenovoTM Venous Stent System 9F: Potential for proximal end of stent not to expand immediately upon deployment for specific product lots (Health Sciences Authority website). 2021. Available at: https://www.hsa.gov.sg/announcements/dear-healthcare-professional-letter/venovo-venous-stent-system-9f-potential-for-proximal-end-of-stent-not-to-expand-immediately-upon-deployment-for-specific-product-lots. Accessed 05/01/2021.
- Recall of VICI and VICI RDSTM Venous Stent Systems due to reports of stent migration after implantation (Health Sciences Authority website). 2021. Available at: https://www.hsa.gov.sg/announcements/dear-healthcare-professional-letter/recall-of-vici-and-vici-rds-venous-stent-systems-due-to-reports-of-stent-migration-after-implantation. Accessed 05/01/2021.
Clinical Topics: Anticoagulation Management, Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Vascular Medicine, Statins, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Echocardiography/Ultrasound, Nuclear Imaging
Keywords: Constriction, Pathologic, Phlebography, Postthrombotic Syndrome, Venous Thrombosis, Thrombectomy, Stents, Lower Extremity, Arteries, Catheters, Thrombolytic Therapy, Pain, Anticoagulants, Ultrasonography, Interventional, Family Characteristics, Phenobarbital
< Back to Listings