Direct to Consumer Wearables For Children: Who, What, When and How?
Quick Takes
- Direct to consumer wearables (D2CWs) are now more readily available. The clinical need must be matched to the device, with shared decision making between patient/parent and physician.
- Patients/parents must understand the workflow of data acquisition and transmission to increase the number of high-yield, actionable tracings.
- D2CW data integration into the electronic medical records is essential to incorporate these technologies into clinical practice.
Introduction
Miniaturization of electronics and sensors has led to increased direct to consumer wearable (D2CW) technologies initially designed for wellness assessment and fitness tracking.1,2 Photoplethysmography (PPG) technology paved the way for arrhythmia monitoring in wearable electrocardiograms (ECGs),3 pulse oximetry assessments,4 and sleep apnea monitoring.5 Continued development of algorithms has increased clinician and patient use of D2CWs for cardiovascular care6 and has also emerged as an important tool in the pediatric population.7,8
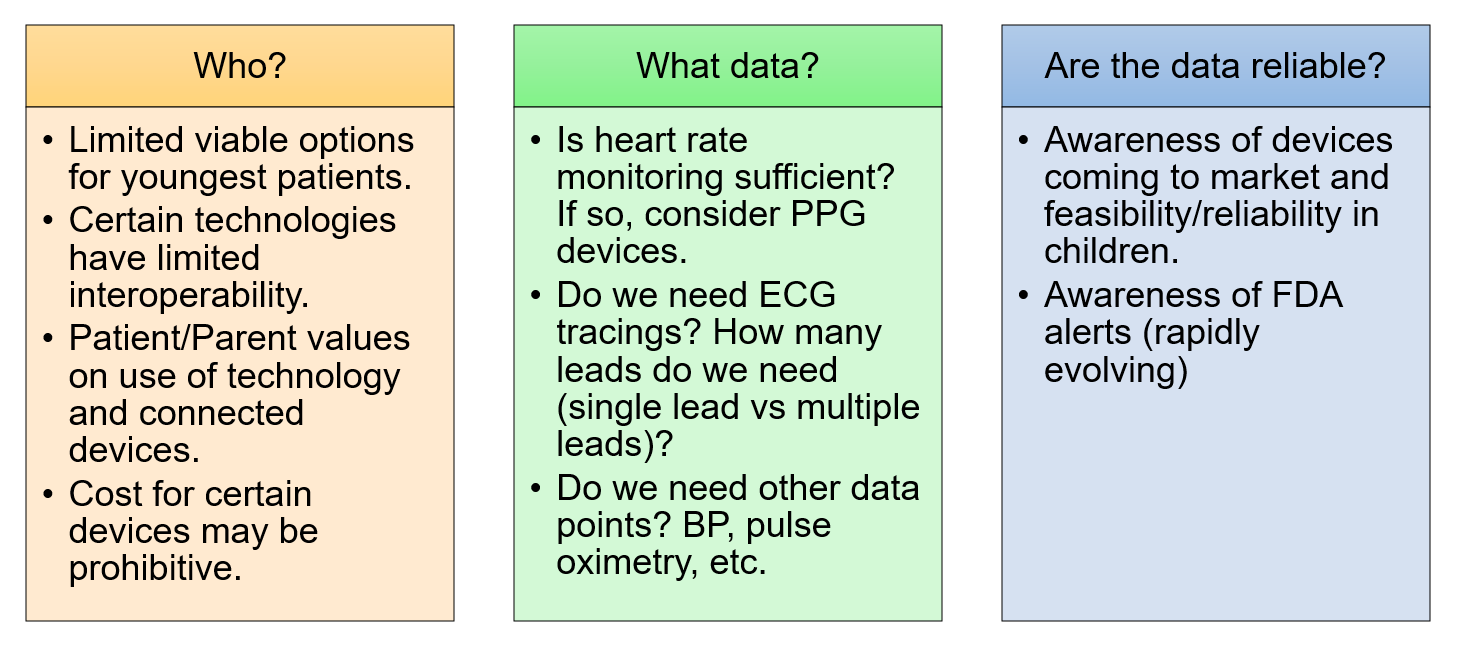
We explore D2CWs and their use in pediatric and congenital heart disease patients — identifying for whom these tools are appropriate, what tools should be considered, how to implement device use, and how to manage acquired data. (Figure 1).
Figure 1
Current Landscape
Approximately 20% of United States (US) residents own a smart wearable device9 and 86% of patients say their device improves their quality of life.10 Large quantities of data are generated from these D2CWs2 in various formats, including HR/pulse tracking, ECG tracings, and activity sensors (Table 1).
Table 1
| Example Wearable Device | Data obtained | FDA cleared | Published Pediatric Data |
| Garments | |||
| AIO SMartSleeve | HR, activity, ECG, sleep | No | No |
| CardioSport | 3-Lead ECG, event detection | No | No |
| Hexoskin | 1-Lead ECG, RPM, VO2 Max, activity, sleep | No | No |
| Nanotube fiber clothing | HR and ECG | No | No |
| Zephyr™ BioHarness 3 clothing | ECG, HR, activity, RR, temperature | Yes | No |
| Jewelry (watches, rings, etc.) | |||
| Apple Watch | ECG, HR, sleep, activity, pulse oximetry | Yes (ECG) | Yes |
| Fitbit (Flex, One, Charge) | ECG, HR | Yes | No |
| Joule Earrings | HR, activity | No | No |
| Omron Heart Guide | HR, activity, sleep, cuff BP | Yes | No |
| Oura Ring | Sleep, HR, activity, temperature | No | Yes |
| Withings ScanWatch, Sleep Mat | BP, HR, ECG, sleep, activity, pulse oximetry | Yes | No |
| Other | |||
| AliveCor® KardiaMobile | ECG – single lead, 6 lead | Yes | Yes |
| Owlet | HR | No | Yes |
| Valencell | HR, HRV, BP, VO2max, pulse oximetry, activity tracking, RR, lactate threshold, RPM | No | No |
Abbreviations: FDA=Food and Drug Administration, HR=heart rate, ECG=electrocardiogram, RPM=remote patient monitoring, BP=blood pressure, HRV=heart rate variability, RR=respiratory rate
Overall, the older pediatric population has a high level of technology savviness, making adoption of these technologies easier in this patient group.
Who May Benefit from D2CWs?
Patients of all ages may benefit from D2CWs11-16 and careful consideration of device type is necessary by age. Assessment of patient/family values about the use of technology by children, including the connected nature of these devices and potential data privacy issues, may impact the types of devices considered.
Appropriate Device Selection
When discussing D2CWs with patients, the clinical question must be identified, and the patient paired with the appropriate technology. Often, patients who ask about D2CWs are acutely aware of their symptoms and desire to understand them better. Empowering patients to have control over their personal data may be useful for promoting behavioral changes and improve overall health.1
Patients and families often look to clinicians for guidance regarding D2CWs.2 Clinicians should engage in shared decision making with patients/families for their specific needs. For instance, when assessing supraventricular tachycardia (SVT) burden in symptomatic teenagers, ECG based technologies may be ideal, allowing discernment between sinus tachycardia versus supraventricular tachycardia. For newborns with documented SVT, simple HR monitors may best help assess arrhythmia burden.
Cost must be considered with device prices ranging from <$100 to >$600. Certain US Food and Drug Administration (FDA) approved devices may be reimbursable through flexible spending accounts (FSAs) or health savings accounts (HSAs).
Other considerations include ease of use, portability, and smartphone connectivity.17 Because the use of D2CWs require an individualized approach, a concise guide would be helpful when determining best fit. One such tool, the ABCD guide is as follows:1 A=assessment of price, regulatory approvals, best practice guidelines, B=benefits to patient/clinical practice, C=clinical workflow, D=data rights, storage, governance, privacy.
Practical Implementation
Once the patient/family decide to move forward with a D2CW, clinicians help them understand data transmission workflow and set expectations for receiving results, with a trial practice of data acquisition. A clinician will walk through the process of transmitting data to the health care team and provide feedback when information is received. Patients are made to understand that this mode of communication is for routine tracings and should never be used in an emergency.
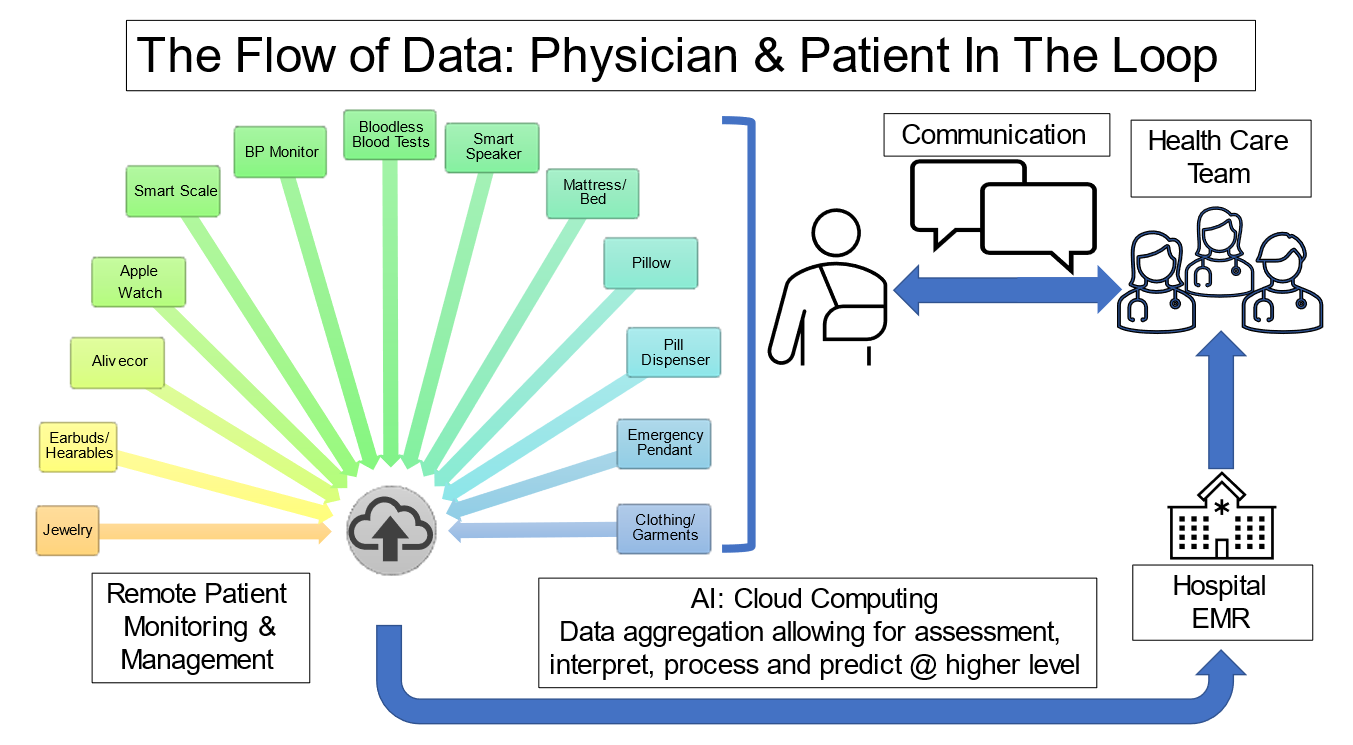
By practicing the patient workflow during "normal" circumstances, the aim is for the patient/family to be familiar with data acquisition and transmission in advance of a clinical event. (Figure 2).
Figure 2
Patient acquired real world data are uploaded and transmitted. Cloud computing with both edge and cloud-based artificial intelligence will allow not only data aggregation, but also higher order interpretation, processing, and event prediction. These data points are ideally entered into the electronic medical record (EMR) and transmitted to the health care team who will develop a patient specific plan which is then communicated back to the patient. The circle is completed when new data points are acquired under new conditions and data is then collected and transmitted, allowing not only for remote monitoring, but remote management as well. Clinicians spend time with patients to help them distinguish between a normal versus abnormal tracing, reinforcing this at each clinic visit, empowering them to be engaged partners and minimizing "worried well" behaviors.
Data Integration to the EMR
It is important to consider EMR integration of data generated by D2CWs. Patient portals are one way that this can be achieved in a safe, HIPAA-compliant format. Clinicians use the patient portal MyChart (Epic Systems, Verona, WI), where patients upload recorded data and send it via secure messaging into their EMR.8 The clinician then determines if additional testing is needed or a change in plan is required. Return communication is also performed through the patient portal, allowing other health care team members to remain in the loop on patient management and communication.
Future Directions
Pediatric cardiac electrophysiology patients are well-suited for the expansion of digital health and D2CWs focusing on identification, management, and screening of arrhythmias. Continued publication of high-quality clinical data is essential for integration into the traditional clinic workflow.18
Emerging technologies include integration of blockchain19 and non-fungible tokens20 to provide greater levels of data encryption, storage, and patient privacy/data ownership. Expanding and securing artificial intelligence systems will aid in diagnosis and management of arrhythmias given the increased data generation. Finally, involvement of digital health companies with cloud-based storage will allow for increased data accessibility for patients receiving care in multiple hospital systems.
References
- Duncker D, Ding WY, Etheridge S, et al. Smart wearables for cardiac monitoring-real-world use beyond atrial fibrillation. Sensors (Basel) 2021;21;2539.
- Tarakji KG, Silva J, Chen LY, et al. Digital health and the care of the patient with arrhythmia: what every electrophysiologist needs to know. Circ Arrhythm Electrophysiol 2020;13:e007953.
- Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a Smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909-17.
- Dagher L, Shi H, Zhao Y, Marrouche NF. Wearables in cardiology: here to stay. Heart Rhythm 2020;17:889-95.
- Fonseca P, Weysen T, Goelema MS, et al. Validation of photoplethysmography-based sleep staging compared with polysomnography in healthy middle-aged adults. Sleep 2017;40:zsx097.
- Ding EY, Svennberg E, Wurster C, et al. Survey of current perspectives on consumer-available digital health devices for detecting atrial fibrillation. Cardiovasc Digit Health J 2020;1:21-29.
- Tandon A, de Ferranti SD. Wearable biosensors in pediatric cardiovascular disease. Circulation 2019;140:350-52.
- Roelle L, Dalal AS, Miller N, Orr WB, Van Hare G, Silva JNA. The impact of direct-to-consumer wearables in pediatric electrophysiology telehealth clinics: a real-world case series. Cardiovasc Digit Health J 2020;1:169-71.
- Bayoumy K, Gaber M, Elshafeey A,. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol 2021;18:581-99.
- Hedges L. Considering the Patient Perspective When Prescribing Medical Wearables (Patient Safety Learning - The Hub). 2022. Available at: https://www.pslhub.org/learn/commissioning-service-provision-and-innovation-in-health-and-care/digital-health-and-care-service-provision/other-health-and-care-software/considering-the-patient-perspective-when-prescribing-medical-wearables-2-march-2022-r6442/. Accessed 06/02/2022.
- Anjewierden S, Humpherys J, LaPage MJ, Asaki SY, Aziz PF. Detection of tachyarrhythmias in a large cohort of infants using direct-to-consumer heart rate monitoring. J Pediatr 2021;232:147-53 e1.
- Gropler MRF, Dalal AS, Van Hare GF, Silva JNA. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS One 2018;13:e0204403.
- Hwang J, Kim J, Choi KJ, Cho MS, Nam GB, Kim YH. Assessing accuracy of wrist-worn wearable devices in measurement of paroxysmal supraventricular tachycardia heart rate. Korean Circ J 2019;49:437-45.
- Girvin ZP, Silver ES, Liberman L. Comparison of AliveCor KardiaMobile Six-Lead ECG with Standard ECG in Pediatric Patients (Research Square).2022. Available at: https://assets.researchsquare.com/files/rs-1631035/v1/7a2259eb-c235-43fd-98c8-f9a6cc196a98.pdf?c=1652194004. Accessed 06/02/2022.
- Paech C, Kobel M, Michaelis A, et al. Accuracy of the Apple watch single-lead ECG recordings in pre-term neonates. Cardiol Young 2021:1-5.
- Kobel M, Kalden P, Michaelis A, et al. Accuracy of the Apple Watch iECG in children with and without congenital heart disease. Pediatr Cardiol 2022;43:191-96.
- Raja JM, Elsakr C, Roman S, et al. Apple watch, wearables, and heart rhythm: where do we stand? Ann Transl Med 2019;7:417.
- Cohen AB, Mathews SC, Dorsey ER, Bates DW, Safavi K. Direct-to-consumer digital health. Lancet Digit Health 2020;2:e163-e65.
- Xiao Y, Xu B, Jiang W, Wu Y. The HealthChain blockchain for electronic health records: development study. J Med Internet Res 2021;23:e13556.
- NFT In Healthcare: How Patients Could Monetise Their Health Data (The Medical Futurist). 2022. Available at: https://medicalfuturist.com/nfts-an-health-data/. Accessed 06/02/2022.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Atrial Fibrillation/Supraventricular Arrhythmias, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Quality Improvement, Sleep Apnea
Keywords: Adolescent, Child, Patient Portals, Cloud Computing, Quality of Life, Artificial Intelligence, Data Aggregation, Decision Making, Shared, Electronic Health Records, Electrophysiologic Techniques, Cardiac, Health Insurance Portability and Accountability Act, Medical Savings Accounts, Photoplethysmography, Privacy, Smartphone, Tachycardia, Sinus, United States Food and Drug Administration, Communication, Electrocardiography, Patient Care Team, Wearable Electronic Devices, Technology, Heart Defects, Congenital, Computer Security, Miniaturization, Oximetry, Ambulatory Care, Sleep Apnea Syndromes, Electronics, Digital Technology, Hospitals
< Back to Listings