Feature | Robotics in Cardiac Intervention: An Update
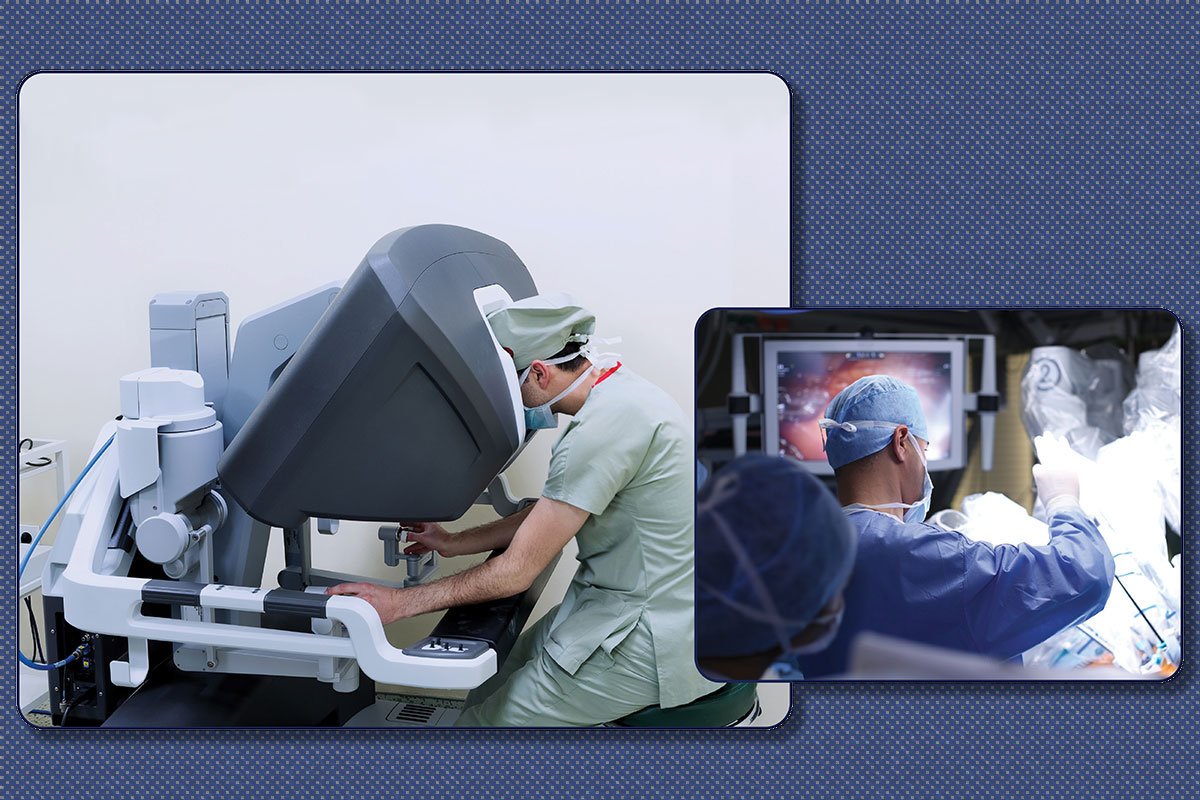
In a world saturated with headlines highlighting breakthroughs in artificial intelligence and virtual reality, robotics may seem somewhat conventional. But, in fact, robotic-assisted solutions for cardiovascular intervention are likely to become more common as market dynamics change, more surgeons learn to use robotic systems, and more evidence of benefit accrues.
"My vision for the future is that robotic surgery of some sort will be a major part of cardiovascular disease care," says W. Randolph Chitwood Jr, MD, FACC, a cardiothoracic surgeon at East Carolina University in Greenville, NC, and an early adopter of robotic cardiac intervention.
"Patients will always prefer the least invasive option. While I'm not sure robotic options across cardiology are advancing uniformly, I think there's still a lot of potential to be explored," he adds.
Da Vinci's Monopoly
 Department of Defense
Department of Defense
Any discussion of surgical robotics necessarily focuses on the da Vinci robotic system, with more than 6,700 systems deployed around the world, almost two-thirds of which are in the U.S.
The robot sells for anywhere up to $2 million, with more than half of the company's $6.5 billion in annual revenue coming from instruments, accessories and service contracts.
The reign of more than two decades of the da Vinci system is likely nearing its end with the expiration of a number of patents in its portfolio.1 "Basically, anyone can copy the da Vinci now, and there are companies in China and India starting to do this. If those systems work well and are approved in the U.S., it will change the field markedly," says Aleksander Dokollari, MD, a surgeon from St. Boniface Hospital in Winnipeg, Manitoba, Canada. He trained in robotic CABG as a fellow at Lankenau Heart Institute in Wynnewood, PA.
Chitwood, who took delivery at his institution in 1999 of the first commercially available da Vinci robotic system in North America, anticipates it is likely to remain the market leader for some time. Although there are potential competitors, none are approved by the U.S. Food and Drug Administration (FDA). And da Vinci's manufacturer is "so far out front, it's unlikely for anyone to catch up soon unless they offer a markedly reduced price or maybe if they develop a robot for a specific operation," he suggests.
Surgically speaking, da Vinci's biggest impact has been in robot-assisted radical prostatectomy (RARP). Today, at least 90% of radical prostatectomies in the U.S. are done robotically, up from <1% in 2003. RARP doesn't appear to improve long-term cancer outcomes appreciably, compared with open or laparoscopic prostatectomy, but patients prefer RARP to experience fewer acute and chronic postoperative complications.2 Surgeons appreciate the ability to remain seated during the two- to four-hour procedure and report enhanced visualization and greater hand and wrist flexibility.
Robotic Mitral Valve Repair
Robotic mitral valve (MV) repair for degenerative mitral regurgitation has been shown in multiple series to deliver excellent mitral repair rates, durability, and safety.3 Currently, about 15% of all procedures are done with robotic assistance (compared to <1% of CABG procedures) and that rate is expected to increase.4
 Department of Defense
Department of Defense
"The robot is a tool. It's a pair of scissors and a needle holder. But with telemanipulation, you become totally ensconced in the operative topography, as if you're walking into the mitral valve. Honestly, with the robot, you can see so much better than with a sternotomy and it just works really well," says Chitwood, who performed the first MV repair in the U.S. in 2000. Thereafter, two FDA safety and efficacy trials led to approval for intracardiac surgery in 2002.
Before his recent retirement from surgery, he was doing five or six robotic MV repairs a week, including "complex" re-do operations.
Certainly, the data support the less invasive approach, particularly in experienced hands. Mori, et al., recently published a propensity-matched analysis of robotic vs. sternotomy approaches from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database that showed similar mortality and morbidity, but shorter hospital stays and less 30-day readmission with the robotic approach.5 Patients who underwent robotic MV repair were also less likely to require conversion to an MV replacement procedure.
"At this point, robotic MV repair has stood the test of time and wins as the least invasive option. It does require commitment from the hospital administration and good training. But I think it will continue to be embraced by more surgeons who want to be able to offer their patients a nonsternotomy option that offers excellent long-term outcomes," says Chitwood.
Robotic CABG
Although it's still the standard treatment for atherosclerotic cardiovascular disease, most surgeons agree it's high time CABG become less invasive.6
Robotic-assisted CABG (RA-CABG) consists of harvesting the left internal thoracic artery (LITA) followed by off-pump LITA-to-left anterior descending (LAD) artery anastomosis through a 4-cm left mini-thoracotomy. Alternately, with totally endoscopic coronary artery bypass, the entire procedure is performed using robotic arms placed through four or five "ports" placed throughout the anterolateral chest wall, with each incision only 5 to 10 mm long.
"Both of these options have been shown to offer some benefits over a median sternotomy in terms of less postop atrial fibrillation (AFib), fewer bleeding complications and less need for transfusion, and, typically, shorter hospital stays. But in terms of hard endpoints like mortality, minimally invasive options do not appear to offer any improvement over sternotomy," says Aaron Spooner, MD, St. Boniface Hospital in Winnipeg. And, of course, patients much prefer to avoid the hassle and pain of a sternotomy.
However, RA-CABG is no shortcut to CABG expertise, he stresses. "The basic principle is that a profoundly good anastomosis is needed to do a patient justice. If you can't do that with an open chest, you don't want to compound the complications and complexity by doing something less invasively."
– W. Randolph Chitwood Jr, MD, FACC
Not having enough surgeons trained in RA-CABG has limited its wider adoption. Today, almost 20 years after its introduction, only about 1% of CABG procedures are robotic-assisted. Also, the limited data available suggest the learning curve is a steep one: experienced surgeons need to perform at least 10 RA-CABG procedures before they can expect stable clinical outcomes,7 while 250 to 500 cases are needed to "achieve mastery."8
Dokollari presented long-term outcomes of RA-CABG at TCT 2023, subsequently publishing his findings.9,10 His research, conducted while he was a fellow at Lankenau, looked at 2,280 consecutive patients who underwent RA-CABG over a 16-year period. In all patients, RA-assisted LITA harvest was followed by LITA-to-LAD manual anastomosis through a 4-cm left thoracotomy. PCI was then performed in non-LAD coronary targets within seven days post RA-LITA-LAD.
During the study period, the mean age increased (from 64.5 to 68.1 years; p<0.0001) and operative time was progressively reduced (from 6.4 to 5.5 hours; p<0.001), while the incidence of conversion to sternotomy and 30-day death remained similar and low (about 1.6% and 1.2%, respectively). Over time, the rates of all-cause death, major adverse cardiac and cerebrovascular events, nonfatal stroke and repeat revascularization with stents fell significantly (p<0.0001).
Chitwood sees the fully robotic CABG option as being unlikely to gain significant traction, but likes the LITA-LAD RA-CABG option with hybrid PCI to manage the right side of the heart.
"The problem with fully robotic CABG is the need to move the heart into various positions to graft multiple arteries. While there are some experts who are able to do multiple grafts robotically and get a good anastomosis, they are few and far between," says Chitwood.
"There are a lot of surgeons who will do a mammary artery takedown with the robot and then graft it to the LAD by hand, avoiding a full sternotomy and the pump. We've seen good results with that approach. But I don't see the fully robotic approach going far with the technology we have now," he adds.
Dokollari also conducted a propensity-matched cost analysis on the Lankenau data. He found that, even with the cost of the robot factored in, total cost was just under $3.3 million (or about $12,300 per patient) lower for RA-CABG compared with conventional CABG.
While they had higher 30-day readmission rates (9.0% vs. 3.4%; p=0.007), RA-CABG patients had significantly shorter hospital stays, spent less time in the OR, required less ICU-level care, had less postop AFib and required less blood products. Multivariate analysis showed the savings were mainly from shorter hospital length of stay with RA-CABG (five vs. seven days).
"I was frankly surprised to find such a large difference," says Dokollari. "There is this sense that RA-CABG is only for rich clinical centers because it takes longer and requires expensive equipment. But we showed there are actually cost savings from using this technology."
"Obviously the cost of the robot and disposables is a concern, but we found the da Vinci pays for itself within one year just doing RA-CABG. If you add on top of that other types of surgeries, such as prostate and general vascular, it can be paid off more quickly," he adds.
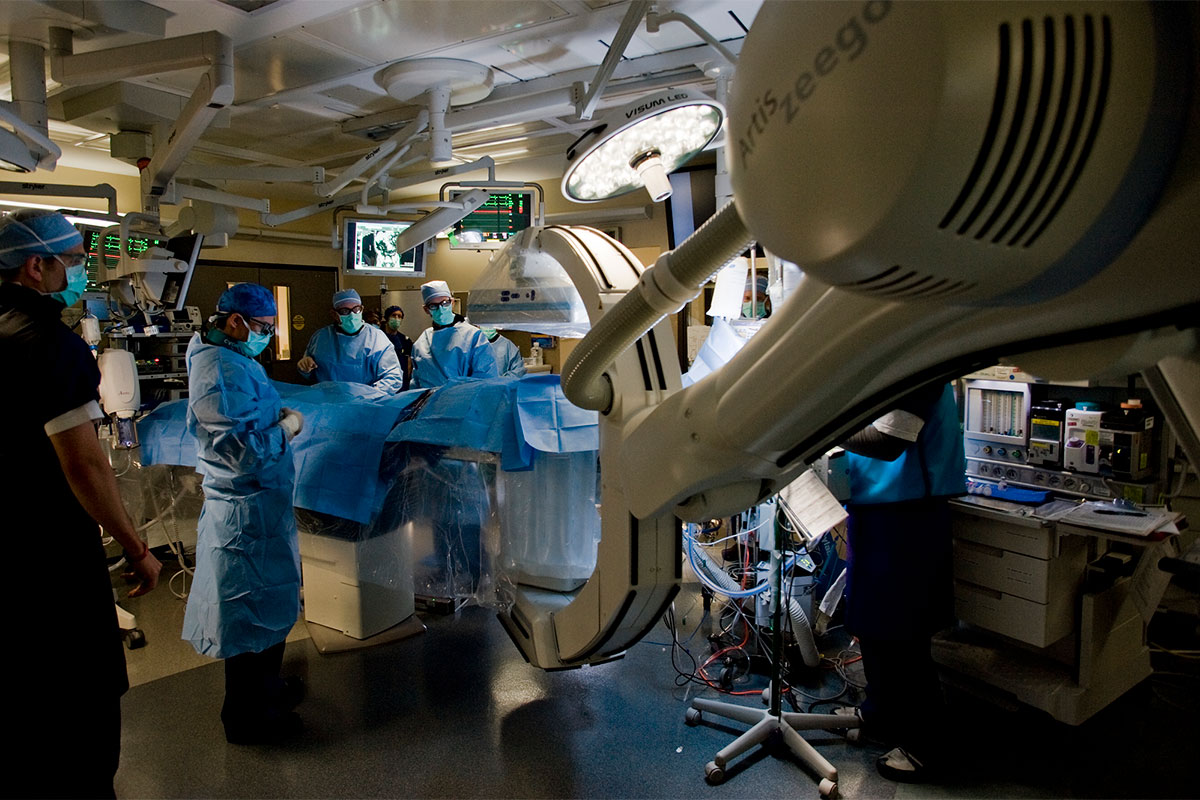
Robotic PCI
 Department of Defense
Department of Defense
Robotic-assisted PCI (R-PCI) is a novel technology that enables control of coronary guidewires and intracoronary devices from a control cockpit, leaving operators protected from radiation exposure and spine injuries related to wearing heavy lead protection. There is also some evidence that it may improve the precision and safety of PCI.11
In R-PCI, arterial access, diagnostic angiography and engagement of the guiding catheter are achieved by traditional means. Once the guiding catheter is engaged, however, operators can remove their lead aprons and position themselves in a remote cockpit to advance, retract and rotate the guidewire with joysticks. Rapid‐exchange angioplasty balloons and stents can be deployed remotely.
"The nurse remains in the cath lab, but only get close to the radiation source if needed to change catheters and such, which of course is not done during fluoroscopy," says Pedro Lemos, MD, PhD, from Hospital Israelita Albert Einstein in Sao Paulo, Brazil. Lemos and his team started operating with the Corindus CorPath GRX robotic system in 2019 and have completed close to 200 cases.
"We were very enthusiastic about it as a means to reduce radiation exposure, which is particularly important for women interventionalists. Then when the pandemic came, we realized we could also use robotic PCI to limit close contact and air-sharing between patients and team members," says Lemos.
Only about 4% of interventional cardiologists are women, notes Sheila Sahni, MD, FACC, an interventional cardiologist and director of the Women's Heart Program at the Sahni Heart Center in New Jersey. "Occupational radiation reduction strategies such as robotic PCI are very attractive to both sexes," she adds.
"The challenge is that the equipment is limited to a small number of U.S. hospitals, limiting access for interventional cardiologists to train adequately on the equipment. And as with any equipment, adoption requires a learning curve which may be difficult for any new graduate of interventional cardiology fellowship to adopt," she says.
Lemos and colleagues have reported an angiographic success rate (successful dilation of the target lesion using robotic assistance) of 99.1% with no safety signals.12 In a small cohort (n=10), they showed that R-PCI could be performed in 100% of cases with the team positioned more than two meters from the patient for ≥50% of the procedure duration and with no in-hospital death or acute target lesion occlusion.13
The program at his hospital, however, has ground to a halt, after Siemens Healthineers, which bought Corindus in 2019, announced in May they plan to discontinue the CorPath GRX robot due to disappointing sales. Now, says Lemos, they are no longer able to obtain the single-use cassettes in which devices, including wires, balloons and stents, are loaded.
With the CorPath robot gone, Robocath's R-One robotic system is the only other commercially available R-PCI system. The R-One received a CE marking in February 2019 and is currently available throughout Europe and Africa. A third device, the Magellan Robotic System (Hansen Medical), is FDA-approved but designed for peripheral vascular intervention. Other systems are in development.14
"We're believers in robotic PCI. We used it, we liked it and we think it will become increasingly important and offer real benefits to the field," says Lemos.
Looking Ahead
Beyond the applications discussed here, telemanipulation technologies are currently being used for benign cardiac tumor excision, atrial septal defect closure, tricuspid valve operations, cryothermic Cox Maze ablation, and in some centers, septal myectomy, aortic valve replacement, and repair of anomalous pulmonary venous connection.15
Chitwood thinks the future, overall, for robotic cardiac intervention is a bright one, albeit with some bumps along the way. "I think in the future, we'll see more training opportunities for surgeons and residents and more patients opting for less invasive options, particularly for the MV, which has been shown to be safe and offer excellent long-term outcomes," says Chitwood.
He adds, "I also think new technologies and innovation will improve surgical robots such that their benefits will be increasingly clear and they will continue to be embraced for a wider array of cardiac operations and pathologies."
This article was authored by Debra L. Beck, MSc.
References
- Edwards G. The Patent Power Struggle in Surgical Robotics. Med-Tech Innovation. Jan. 3, 2020. Available here. Accessed Nov. 1, 2023.
- Wu SY, Chang CL, Chen CI, Huang CC. Comparison of acute and chronic surgical complications following robot-assisted, laparoscopic, and traditional open radical prostatectomy among men in Taiwan. JAMA Netw Open 2021;4:e2120156.
- Chikwe J, Trento A, Cheng W, et al. Commentary: Lessons from 1000 robotic mitral repairs. J Thorac Cardiovasc Surg 2021;161:94-5.
- Balkhy HH. Achieving mastery in robotic-assisted coronary artery bypass surgery: Why stop at one internal thoracic artery graft!? Ann Thorac Surg 2023;115:1125-6.
- Mori M, Parsons N, Krane M, et al. Robotic mitral valve repair for degenerative mitral regurgitation. Ann Thorac Surg 2023;Aug 16:S0003-4975(23)00852-4.
- Ruel M. Designing the coronary artery bypass surgery operation of the future. Curr Opin Cardiol 2023;38:490.
- Patrick WL, Iyengar A, Han JJ, et al. The learning curve of robotic coronary arterial bypass surgery: A report from the STS database. J Card Surg 2021;36:4178-86.
- Jonsson A, Binongo J, Patel P, et al. Mastering the learning curve for robotic-assisted coronary artery bypass surgery. Ann Thorac Surg 2023;115:1118-25.
- Dokollari A, Sicouri S, Erten O, et al. Long-term clinical outcomes of robotic-assisted surgical coronary artery revascularisation. Eurointervention 2023;Nov 23:EIJ-D-23-00373.
- Dokollari A, Sicouri S, Pendegrast G, et al. Robotic-assisted vs traditional full-sternotomy coronary artery bypass grafting procedures. A propensity-matched analysis of hospital costs. Am J Cardiol 2023;Nov 26:S0002-9149(23)01259-6.
- Weisz G, Smilowitz NR, Metzger DC, et al. The association between experience and proficiency with robotic-enhanced coronary intervention-insights from the PRECISE multi-center study. Acute Card Care 2014;16:37-40.
- Lemos PA, Franken M, Jr JM, et al. Safety and effectiveness of introducing a robotic-assisted percutaneous coronary intervention program in a tertiary center: A prospective study. Cardiovasc Diagn Ther 2022;12:67-76.
- Lemos PA, Franken M, Mariani J, et al. Use of robotic assistance to reduce proximity and air-sharing during percutaneous cardiovascular intervention. Future Cardiol 2021;17:865-73.
- Indolfi C, Polimeni A, De Rosa S, Danieli G. First-in-human robotic percutaneous coronary intervention by the ROSES robotic system: a case report. Eur Heart J Case Rep 2023;7(10):ytad506.
- Badhwar V, Wei LM, Geirsson A, et al. Contemporary robotic cardiac surgical training. J Thorac Cardiovasc Surg 2023;165:779-83.
Clinical Topics: Valvular Heart Disease, Mitral Regurgitation
Keywords: Cardiology Magazine, ACC Publications, Robotic Surgical Procedures, Mitral Valve Insufficiency, Artificial Intelligence, Cardiovascular Diseases

