Cover Story | New Frontiers in Heart Failure Care: Innovations Reshaping Treatment

The idea that obesity increases the risk of heart failure seems like an obvious conclusion. After all, as the Framingham Heart Study clearly showed, obesity and overweight are significantly associated with a higher risk of hypertension, angina and coronary heart disease.1
In addition, up to 80% of people with the most common form of the condition, heart failure with preserved ejection fraction (HFpEF), have overweight or obesity.2-4
Yet for decades, says Mikhail Kosiborod, MD, FACC, who co-directs the Cardiometabolic Center of Excellence at St. Luke's Hospital in Kansas City, MO, there was a belief that when it came to HF, "bigger was better," with even mild overweight associated with a significant benefit in terms of morbidity and mortality.
The belief stemmed from epidemiological studies showing that patients with HF who lost weight had a higher mortality rate and were more likely to require transplant.3 Those observational studies, however, didn't distinguish between unintentional and intentional weight loss and predominantly focused on heart failure with reduced ejection fraction (HFrEF), he says.
Now, however, the results of two large studies showing the stunning benefits of the GLP-1 receptor agonist (GLP-1 RA) semaglutide, an anti-diabetes and anti-obesity medication, in patients with HFpEF has shattered the obesity paradox.
– Mikhail Kosiborod, MD, FACC
The pivotal trials, STEP-HFpEF and STEP-HFpEF DM, were international, multicenter, double-blind, placebo-controlled trials in individuals with the obesity phenotype of HFpEF. Both studies randomized patients to once weekly semaglutide 2.4 mg or placebo.
Primary endpoints were mean change in the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) and percentage change in body weight. Secondary endpoints were change in the six-minute walk distance (6MWD); a hierarchical composite endpoint that included death, HF events and differences in the change in the KCCQ-CSS and 6MWD; and change in C-reactive protein (CRP) level.4,5
Both trials demonstrated significantly improved HF symptoms and physical limitations as well as weight loss; enhanced functional capacity; and reduced inflammatory markers compared with placebo in patients with and without type 2 diabetes (T2D) (Tables 1 and 2). The most common adverse events in both trials were gastrointestinal.
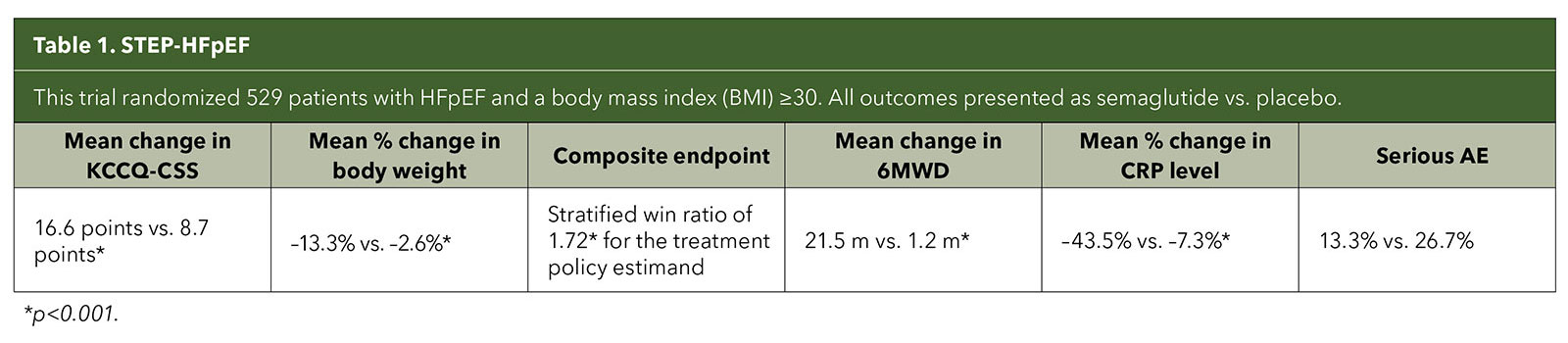
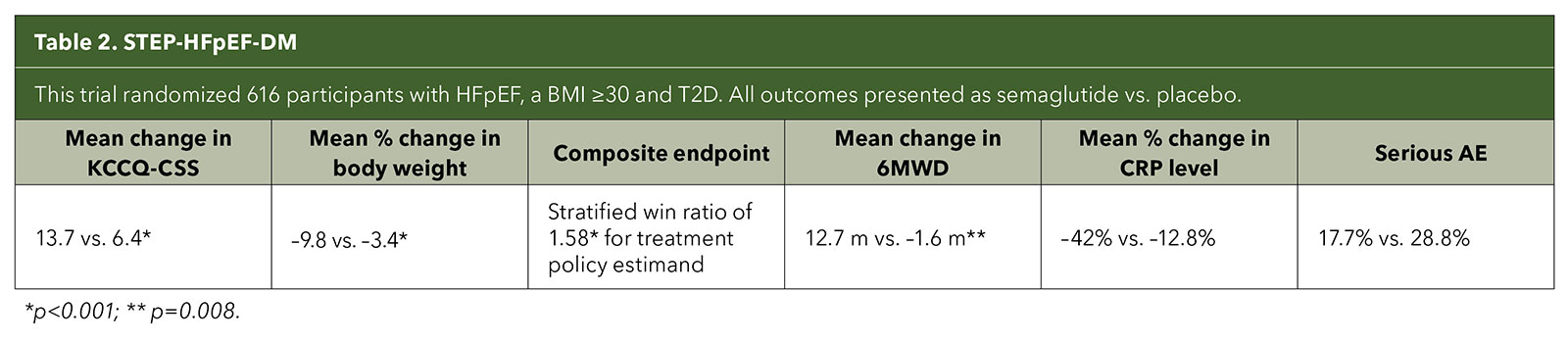
Although the trials weren't designed to assess long-term outcomes like cardiovascular deaths and HF hospitalizations, when it came to the primary endpoints of improvement in symptoms and physical limitations due to HF, the magnitude of the trial results surprised even the STEP-HFpEF investigators.
"It was a bit of a shock to the clinical community because this was not expected," says Kosiborod, who was the principal investigator of both studies. "People knew we were doing a trial, but I think a lot of clinicians were questioning this particular approach because of this pervasive notion that weight loss is not something we do in heart failure. In fact, maybe it's a bad thing."
"I don't think most people in the clinical community were expecting this trial to be as positive as it ended up being," he says. "It's not just that the trials were positive, but the treatment effect was so large that it was, in a way, paradigm shifting."
According to Brian Hsi, MD, FACC, who specializes in advanced HF and transplant at Baylor University Medical Center in Dallas, TX, the trial results have caused a change in thinking about obesity as simply a mechanical cause of people having shortness of breath and symptoms of HF to talking about direct causes and consequences from obesity that lead to heart dysfunction.
"It just shows," says Kosiborod "that we've got to question conventional wisdom and we have to do randomized control trials to test hypotheses."
Testing the Hypothesis
The STEP-HFpEF trials were designed based on intriguing observations from Kosiborod's Cardiometabolic Center of Excellence, which delivers integrated care for people with obesity, diabetes and cardiovascular disease.
"We ended up using a lot of medications like semaglutide in those patients, not as a treatment for HF and not necessarily for weight loss, but because the drug previously had been shown to reduce the risk of things like heart attacks and strokes in people with diabetes," he says.
The team found that not only did patients lose weight and improve their diabetes control while on semaglutide, but their HF symptoms also improved.
"It wasn't just one patient or two patients," says Kosiborod. "It was the whole series of patients. At some point you realize it's not an accident; that what you're observing may well be a clear effect of the medication on heart failure."
Moving into Clinical Practice
Hsi has already started using GLP-1 RAs in his practice and says many of his colleagues are as well. "Just like with the SGLT2 inhibitor class of medications, more cardiologists are seeing them not as an endocrinology drug but rather as something in the cardiovascular space that should help improve patient outcomes."
However, he stresses, the drug should not be the first option for everyone with obesity and HF.
"Body weight should absolutely be a treatment target for patients," he says, "and if the difference between the patient's current weight and ideal, or at least reasonable, body weight is too great, we should look beyond medical therapies like GLP-1s and into metabolic surgery."
"If it takes 12 months to lose 5% of your body weight and I'm telling you that you have six months to live, you need something that's been proven effective for higher amounts of weight loss," he says. In fact, studies consistently find patients lose more weight faster with bariatric surgery than with weight loss medications. The weight loss with bariatric surgery is also sustained longer overall than with medications, he says.
"Even within the advanced HF population, bariatric surgery is absolutely an option," Hsi says. "We're able to get patients safely through bariatric surgery all the way to something like using temporary mechanical support." Studies also find that bariatric surgery, even in patients with severe HF, can significantly reduce the risk of mortality, decrease hospital admissions and length of stay, reduce exacerbations, and improve quality of life.
For patients who do qualify for a GLP-1 RA, Hsi has a unique approach to addressing the gastrointestinal side effects that often occur – and that may lead to discontinuation – when the drugs are started.
"I don't really sell it to them as a side effect," he says. "I explain that the GLP-1 RAs mimic a hormone in the body that tells them that they're full and should stop eating." And that if they continue to eat despite what their brain is telling them to do, then their brain is going to activate mechanisms that tells them to stop, like vomiting.
Digging Deeper into the Results
Since the results of the first STEP-HFpEF study were announced in 2023, several prespecified subanalyses of both trials have been published, adding excitement to the story of semaglutide and HF. Among the findings:
- An analysis of STEP-HFpEF found the greater the weight loss, the greater the improvement in primary and secondary endpoints.6
- The benefits of treatment in the STEP-HFpEF trial, including weight loss, were consistent across the range of patients with moderately reduced and preserved ejection fraction.7 This raises the intriguing possibility that semaglutide may also be effective in patients with other forms of HF.
- Pooled data from both trials found that although women (who made up about half of participants) had a higher BMI, left ventricular ejection fraction, CRP and worse HF symptoms at baseline, those treated with semaglutide demonstrated similar KCCQ-CSS as men as well as greater body weight reduction. Improvements in the 6MWD and hierarchical composite endpoint were similar between both sexes.8
- About half of participants in the two trials had atrial fibrillation (AFib), which was associated with more advanced HF. Those with AFib treated with semaglutide experienced a greater magnitude of improvement in HF-related symptoms and physical limitations than those without AFib at baseline.9
- Echocardiography performed in 43% of participants in the two trials found semaglutide appeared to improve adverse cardiac remodeling compared with placebo. This, the investigators wrote, suggests that treatment with semaglutide may be disease modifying.10
- Semaglutide-treated patients had greater CRP reductions compared with placebo regardless of their baseline level or the amount of weight loss.11
- Pooled data from both trials found 32.6% of semaglutide-treated patients had an improvement in NYHA functional class vs. 21.5% with placebo, and just 2.09% experienced any deterioration compared with 5.24% with placebo.12
- Semaglutide significantly reduced loop diuretic requirements.13
Neither of the STEP-HF trials had hospitalization or mortality as endpoints, but a meta-analysis of those trials and two others looking at semaglutide in cardiovascular disease (SELECT and FLOW) provide some clues.
The SELECT trial enrolled participants with atherosclerotic cardiovascular disease and overweight or obesity, and the FLOW trial enrolled participants with T2D and chronic kidney disease. Across the four trials, which enrolled 22,282 participants, 16.8% also had HFpEF.
In the analysis, semaglutide significantly reduced the risk of the combined endpoint of cardiovascular death or HF events compared with placebo (5.4% vs. 7.5%; hazard ratio [HR], 0.69; 95% CI, 0.53-0.89) and reduced the risk of worsening HF events from 4.7% in the placebo group to 2.8% in the semaglutide cohort (HR, 0.59; 95% CI, 0.41-0.82; p=0.0019).
In addition, fewer semaglutide-treated patients experienced any serious adverse events compared with placebo (29.9% vs. 38.7%).14
Beyond Weight Loss: Unraveling the Mechanisms
Is it a weight loss drug or is it a HF drug? This is one of the most common questions when presenting the trial results with semaglutide says Kosiborod. In other words, "are the benefits from the mechanical unloading from weight loss or is this actually disease-modifying therapy for HF?"
Most likely it's both, he says. First, the HF benefits were the same regardless of the amount of weight loss. He also points to the significant reduction in N-terminal pro-B-type natriuretic peptide (NT-proBNP), which is released from the myocardium in response to congestion-related stretch, in the semaglutide groups.
– Brian Hsi, MD, FACC
Studies in patients with HF who undergo bariatric surgery find those levels go up, not down. And, interestingly, in the STEP-HFpEF trials those with the greatest weight loss demonstrated the lowest reduction in NT-proBNP levels, suggesting effects on HF pathobiology unrelated to weight loss.15
The pooled analysis that included SELECT and FLOW participants also showed that those with indicators of more advanced HF (higher natriuretic peptides, higher NYHA functional class, requirement for loop diuretics) also experienced greater improvements in KCCQ-CSS even though they lost a similar degree of weight as those with less severe HF.15
"Clearly, what we've shown in multiple studies since presenting the original results is that this is not just mechanical unloading from weight loss, because while weight loss is likely an important component of the benefit, it cannot explain everything that we're observing," adds Kosiborod.
"It's hard to know exactly why this happens or what the interplay is," says Hsi. "But bottom line, what's most important for patients is do they live better. And I think that's the most important takeaway for the results from these data."
Next Steps
Learn More
Click here to read the articles from the STEP-HF program published simultaneously in JACC with their presentation at ESC Congress 2024, along with video interviews with Kosiborod and Krumholz.
Click here for JACC's new comprehensive obesity hub highlighting the latest research, commentary and multimedia, including a special collection of obesity clinical trials.
The story of GLP-1 RAs and HFpEF is just beginning. "There are a lot of anti-obesity medications being developed," says Kosiborod, including double and triple incretin agonists that can lead to greater weight loss. One, tirzepatide, which is approved by the U.S. Food and Drug Administration (FDA) for the treatment of diabetes and obesity, targets the GLP-1 receptor as well as the GLP receptor.
Its manufacturer recently announced the results of the first study of tirzepatide in HF. SUMMIT was a multicenter, randomized, double-blind, parallel, placebo-controlled phase 3 study of 731 participants in 10 countries comparing the efficacy and safety of tirzepatide to placebo in adults with HFpEF and obesity, with or without T2D.19
It assessed three doses of the drug with a median follow-up of 104 weeks, twice as long as the STEP-HFpEF trials. Tirzepatide reduced the risk of adverse HF outcomes (urgent HF visit, HF hospitalization, oral diuretic intensification, and cardiovascular death) by 38% compared with placebo (HR, 0.62; 95% CI, 0.41-0.95; p=0.026).
Participants receiving tirzepatide also demonstrated significant improvements by 52 weeks on the KCCQ-CSS compared with placebo (19.5% vs. 12.7%); improved exercise capacity as measured by the 6MWD; CRP reduction; and body weight reduction (13.9% vs. 2.2%). As of late September, the results had not yet been published or presented.
Both Hsi and Kosiborod suspect the benefits of incretin drugs like semaglutide and tirzepatide are a class effect, but stress that studies are needed to confirm this. Future research also needs to consider the effects of the drugs on long-term outcomes like hospitalization and death; comparisons between different GLP-1 RAs and other anti-obesity medications; the potential benefits in HFrEF; and combination strategies, particularly with SGLT2 inhibitors.
There is already some evidence of enhanced benefits in patients who start a GLP-1 RA while already taking an SGLT2 inhibitor. A retrospective cohort study with 7,044 patients in each cohort found a significantly lower risk of HF exacerbations, all-cause emergency department visits/hospitalizations, new-onset atrial arrhythmias, new-onset acute kidney injury and pulmonary hypertension in the GLP-1 plus SGLT2 cohort compared with the SGLT2-only cohort regardless of BMI, ejection fraction or natriuretic peptide levels.20
"This class of medications represents a transformative addition to the medical armamentarium," says Harlan Krumholz, MD, SM, FACC, editor-in-chief of JACC, "offering a significant opportunity to address obesity and improve cardiometabolic health."
However, he says, 'while the benefits are promising, there remain challenges, especially regarding equitable access and long-term safety." In addition, he adds, more research is needed to understand the full scope of their effectiveness, particularly in diverse patient populations and across longer time horizons.
Editors' Note: Novo Nordisk, which manufacturers semaglutide, submitted an application to the FDA for approval of semaglutide in HF in January 2024, but pulled the application in August and plans to resubmit early in 2025.21 In September, the European Medicines Agency approved additional labeling for the use of semaglutide for improving HF-related symptoms and physical limitations in people with obesity-related HFpEF.22
References
- Wilson PWF, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: The Framingham Experience. Arch Intern Med 2002;162:1867-72.
- Liu C, Udeshi E, Jones D, et al. Abstract 11443: Metabolic Profile Signature of Obesity in Heart Failure With Preserved Ejection Fraction. Circulation 2023;148(Suppl_1):A11443-A11443.
- Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis 2018;61(2):151-56.
- Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023;389:1069-84.
- Kosiborod MN, Petrie MC, Borlaug BA, et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med 2024;390:1394-1407.
- Borlaug BA, Kitzman DW, Davies MJ, et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nat Med 2023;29:2358-65.
- Butler J, Abildstrøm S, Borlaug B. et al. Semaglutide in patients with obesity and heart failure across mildly reduced or preserved ejection fraction. J Am Coll Cardiol 2023;82:2087-96.
- Verma S, Butler J, Borlaug BA, et al. Efficacy of semaglutide by sex in obesity-related heart failure with preserved ejection fraction: STEP-HFpEF trials. J Am Coll Cardiol 2024;84:773-85.
- Verma S, Butler J, Borlaug BA, et al. Atrial fibrillation and semaglutide effects in obesity-related heart failure with preserved ejection fraction: STEP-HFpEF program. J Am Coll Cardiol 2024;Aug 29: Epub ahead of print doi:10.1016/j.jacc.2024.08.023.
- Solomon SD, Ostrominski JW, Wang X, et al. Effect of semaglutide on cardiac structure and function in patients with obesity-related heart failure. J Am Coll Cardiol 2024;Aug 29:Epub ahead of print: doi:10.1016/j.jacc.2024.08.021.
- Verma S, Petrie MC, Borlaug BA, et al. Inflammation in obesity-related HFpEF: The STEP-HFpEF program. J Am Coll Cardiol 2024;Aug 28:Epub ahead of print: doi:10.1016/j.jacc.2024.08.028.
- Schou M, Petrie MC, Borlaug BA, et al. Semaglutide and NYHA functional class in obesity-related heart failure with preserved ejection fraction: The STEP-HFpEF program. J Am Coll Cardiol 2024;84:247-57.
- Shah SJ, Sharma K, Borlaug BA, et al. Semaglutide and diuretic use in obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials. Eur Heart J 2024;45:3254-69.
- Kosiborod MN, Deanfield J, Pratley R, et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: a pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet 2024;404:949-61.
- Petrie MC, Borlaug BA, Butler J, et al. Semaglutide and NT-proBNP in obesity-related HFpEF: Insights from the STEP-HFpEF program. J Am Coll Cardiol 2024;84:27-40.
- Sarma S, Palcu P. Weight loss between glucagon‐like peptide‐1 receptor agonists and bariatric surgery in adults with obesity: A systematic review and meta‐analysis. Obesity 2022;30:2111-21.
- Aleassa EM, Khorgami Z, Kindel TL, et al. Impact of bariatric surgery on heart failure mortality. Surg Obes Relat Dis 2019;15:1189-96.
- Mottel BH, Lindsay DA, Frishman WH. Effect of bariatric surgery on cardiovascular function and heart failure outcomes. Cardiol Rev 2021;29:187-94.
- Lilly's tirzepatide successful in phase 3 study showing benefit in adults with heart failure with preserved ejection fraction and obesity. August 1, 2024.
- Patel R, Wadid M, Makwana B, et al. GLP-1 receptor agonists among patients with overweight or obesity, diabetes, and HFpEF on SGLT2 inhibitors. JACC Heart Fail 2024;Aug 16:[Epub ahead of print: doi:10.1016/j.jchf.2024.07.006.
- Dunleavy K. Novo Nordisk pulls its FDA heart failure submission for Wegovy, will reapply early next year. Fierce Pharma. Aug 7, 2024. Available here.
- Novo Nordisk A/S: Wegovy® recommended by the European regulatory authorities for label update to reflect reduced heart failure symptoms and improved physical function. NP Investor. Sept 19, 2024. Available here. Accessed Sept 30, 2024.
Clinical Topics: Heart Failure and Cardiomyopathies, Prevention, Acute Heart Failure, Hypertension
Keywords: Cardiology Magazine, ACC Publications, Heart Failure, Obesity, Glucagon-Like Peptide 1, Hypertension, Coronary Disease, Weight Loss

