Omny-IRE: Novel OMNYPULSE Catheter Effective, Safe in Treatment of Paroxysmal AFib
The OMNYPULSE pulsed field ablation (PFA) catheter had a 100% acute success rate, paired with a strong safety profile, in treating paroxysmal atrial fibrillation (AFib), according to the three-month results of the Omny-IRE trial presented as a late-breaking trial at HRS 2025 and simultaneously published in JACC: Clinical Electrophysiology. Additionally, prespecified remapping at three months following ablation showed good durability of pulmonary vein isolation (PVI).
The single-arm first-in-human safety and effectiveness study conducted at 13 centers in Europe and Canada prospectively enrolled 136 patients with symptomatic paroxysmal AFib (mean age 60 years, 30% women, mean CHA2DS2-VASc score 1.6) to PFA with the OMNYPULSE focal PFA catheter. The novel platform is a large-tip focal, multielectrode, contact force (CF)-sending catheter with 3D electroanatomic mapping integration used in conjunction with the TRUPULSE Generator; 21 operators performed the PFA.
The median procedure times for total procedure, left atrium dwell, total ablation and total fluoroscopy were 105.5, 70.0, 46.9 and 5.0 minutes, respectively. Median PFA time was 176 seconds.
Results showed that for the primary efficacy endpoint of electrical isolation of clinically relevant targeted PVIs, confirmed by entrance block following adenosine/isoproterenol challenge, was effective in all veins (545/545) in all patients (136/136). During the procedure, 16 (3%) of the targeted veins in 10 (7%) patients needed touchup after acute reconnection.
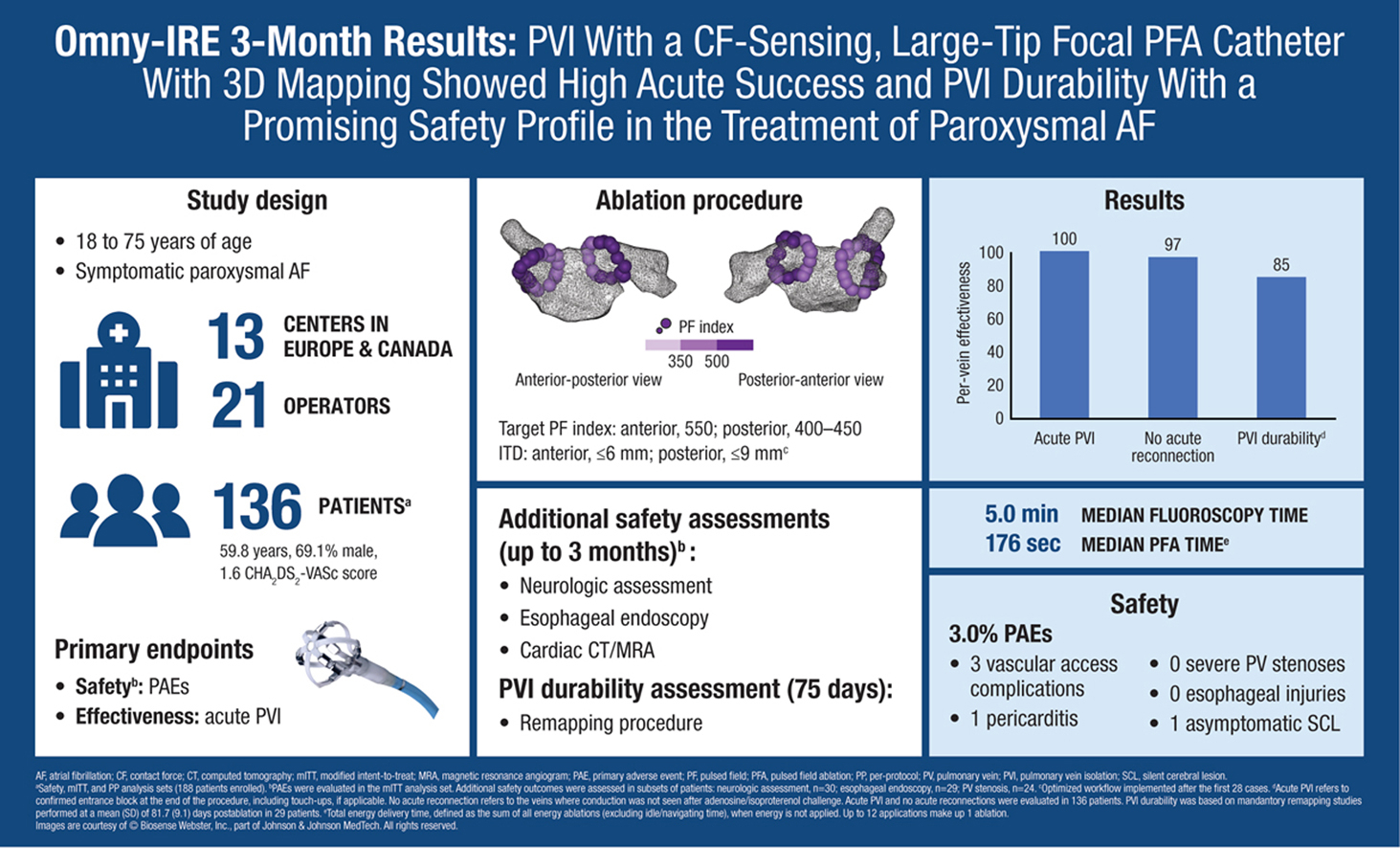
The primary safety endpoint was primary adverse events (PAEs) within seven days of the PFA, defined as major vascular access complication or bleeding, myocardial infarction, pericarditis, pulmonary edema (respiratory insufficiency), phrenic nerve paralysis, stroke or cerebrovascular accident, transient ischemic attack, thromboembolism, heart block, vagal nerve injury, or gastroparesis; cardiac tamponade or perforation (up to 30 days post procedure); and PV stenosis (≥70% reduction in PV diameter), atrioesophageal fistula, and device- or procedure-related death (up to 90 days post procedure).
The primary safety endpoint occurred in four of the 135 patients (3%) in the modified intention to treat analysis; three were major vascular access complications and one had pericarditis, with all determined to be related to the procedure. At three months, there were no device- or procedure-related deaths.
During further safety assessment within subsets of patients, one patient (1/30) was found to have an asymptomatic silent cerebral event at discharge, which resolved at one month without neurological change. There was no observed esophageal injury or PV narrowing >70% (0/24) at three months.
During remapping at 75 days, PVI was found to be durable in 85% of veins (98/116) in 62% of patients (18/29). Within an optimized workflow, durability rose to 89% (75/84) of veins in 71% (15/21) of patients.
"Increasing efficacy after workflow improvements, mainly by decreasing the [intertag distance] on the anterior wall and requiring higher CF, provided clinical evidence of the need for a tighter lesion set and better CF to achieve deeper lesions in thicker tissues," write Mattias Duytschaever, MD, et al.
Regarding the effectiveness of the OMNYPULSE device, the authors add, "This large-focal PFA catheter may be suitable for PVI regardless of the PV anatomy and for ablating lines outside the PVs." They also note that it may support anatomical procedures where the catheter allows the operator to deploy lines and then map block, and may be useful in more complex patient-specific procedures, including "catheter mapping of low-voltage zones, dispersion, and atrial tachycardia to first pinpoint the origin of the arrhythmia then ablate that target."
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Atrial Fibrillation, Pulmonary Veins, Catheters, Electrophysiology
< Back to Listings
