2020 FDA Guidance for Diabetes Drug Development: Cardiorenal Populations and Outcomes: Lessons Learned and Future Directions
Background
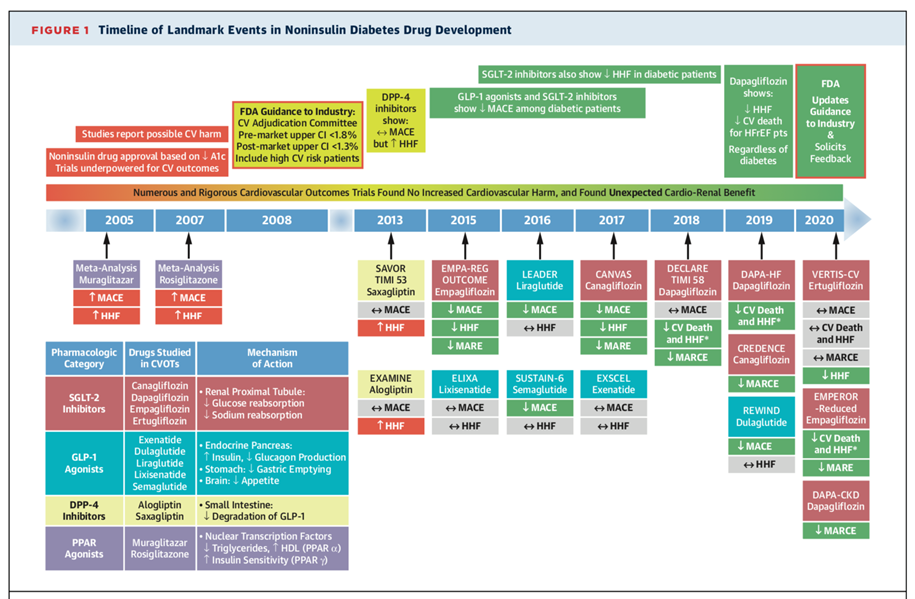
The 2008 Food and Drug Administration (FDA) guidance established new recommendations to evaluate the cardiovascular safety of glucose-lowering drugs, which led to several rigorous cardiovascular outcome trials (CVOTs) to rule out increased cardiovascular risk for all glucose-lowering therapies undergoing evaluation.1 Reassuringly, none of the CVOTs identified an increased risk of ischemic cardiovascular events. Additionally, several trials subsequently showed a positive impact of glucose-lowering drugs on cardiorenal outcomes, which were not originally specified as the primary endpoint of the trials. The most remarkable discovery has been the substantial and consistent risk reduction in cardiorenal outcomes seen across trials of sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs). Based on these considerations, the proposed 2020 FDA guidance recommends an updated approach in the evaluation of the safety profile of these drugs to improve glycemic control in patients with type 2 diabetes mellitus (T2DM).2
CVOTs since 2008 Guidance
The completed trials conducted in response to the 2008 regulatory guidance in patients with T2DM have examined various classes of glucose-lowering therapies, including dipeptidyl peptidase-4 (DPP-4i) inhibitors, GLP-1 RAs and SGLT2i. The main components of the 2008 FDA recommendations included: (1) inclusion of major adverse cardiovascular events (MACE) as primary composite endpoints (cardiovascular mortality, nonfatal myocardial infarction [MI], nonfatal stroke); (2) inclusion of patients at higher cardiovascular risk (for example elderly, advanced cardiovascular disease [CVD] and some degree of renal impairment); (3) meeting an upper boundary for the 95% confidence interval of the MACE hazard ratio (HR) of <1.8 (preliminary marketing approval) and <1.3 (post-marketing trials); (4) adjudication of CV events in a blinded, independent process. Most of the CVOTs adopted the 3-point MACE (cardiovascular death, nonfatal MI, or nonfatal stroke) as the primary outcome, and did not include hospitalizations for heart failure (HHF) or progression of chronic kidney disease (CKD). HHF is a major cardiovascular complication among patients with T2DM;3 moreover, trials that evaluated DPP-4i showed an increased HHF risk in the experimental arms.DECLARE-TIMI 58 added cardiovascular death or HHF as a dual coprimary outcome in addition to 3-point MACE and, subsequently, the EMPA-REG OUTCOME trial similarly added HHF to create a 4-point MACE primary outcome. The primary outcome of the CREDENCE trial was a composite of end-stage kidney disease (dialysis, transplantation, or a sustained estimated glomerular filtration rate of <15 mL·min−1·1.73 m−2), a doubling of the serum creatinine level, or death resulting from renal or cardiovascular causes.
SGLT2i and GLP-1 RAs Trials in Patients with T2DM
Since 2015, results from SGLT2i and GLP-1 RAs trials have consistently shown a significant reduction in MACE among patients with T2DM. Furthermore, trials of SGLT2i went on to show significant reduction in the risk of HHF, namely EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58. More recently, the DAPA-HF and EMPEROR-Reduced trials showed that SGLT2i reduced the risk of cardiovascular mortality, worsening heart failure events and renal outcomes in patients with heart failure with reduced ejection fraction (HFrEF), irrespective of diabetes status.4,5 For renal outcomes, the SGLT2i trials CREDENCE, EMPA-REG OUTCOME, DECLARE-TIMI 58, CANVAS, and DAPA-CKD have shown that SGLT2i use was associated with a reduced risk for adverse renal events compared to placebo. Although GLP-1 RAs have been consistently associated with significant reduction in MACE (as demonstrated in the LEADER, SUSTAIN-, and REWIND trials), individual GLP-1 trials have not demonstrated beneficial effect on HF or kidney outcomes. However, results from a systematic review and meta-analysis in 2019 have shown that treatment with GLP-1 RAs was associated with reduced risk of MACE, HHF and a reduction in the composite kidney outcome (new-onset macroalbuminuria, increase in serum creatinine or greater decline in estimated glomerular filtration rate [eGFR], progression to end-stage kidney disease, or death due to kidney causes), driven mainly by a reduction in urinary albumin excretion.6
Figure 1
2020 FDA Draft Guidance Recommendations
In March 2020 the FDA draft guidance recommended a broader approach to conducting safety evaluations for new glucose-lowering drugs that looks beyond CV risk.2 The draft guidance recommends: (1) at least 4,000 patient-years of exposure to the new drug in phase 3 clinical trials; (2) at least 1,500 patients exposed to the new drug for at least 1 year; and (3) at least 500 patients exposed to the new drug for at least 2 years. The FDA further recommends including patients with underlying CV disease, chronic kidney disease, and older patients: (1) at least 500 patients with stage 3/4 chronic kidney disease exposed to the new drug; (2) at least 600 patients with established CV disease; (3) at least 600 patients aged >65 years exposed to the new drug; and (4) at least 1,200 patients with at least one of these conditions. Patients with T2DM often have comorbid conditions and/or diabetes-associated complications. HF is a major cause of morbidity and mortality in patients with T2DM and CVD. The 2020 draft guidance proposes post-marketing surveillance based on pre-marketing safety signals rather than the mandatory "one-size-fits-all" approach established in the 2008 guidance which recommended meeting an upper boundary for the 95% confidence interval for the estimated risk ratio of <1.8, prior to approval and <1.3 in post-marketing trials. Therefore the 2020 guidance proposes postmarketing requirements based on safety signals identified in the pre-marketing development program promoting flexibility in post-marketing surveillance of diabetes drug development.
Summary and Future Directions
The 2020 FDA draft guidance recommends strategies to optimize diabetes drug evaluation while maintaining the pipeline considering the CVOT results with new classes of glucose-lowering therapies from the past decade. The guidance recommends enrollment of a broader range of patients with comorbidities and diabetes associated conditions including patients with underlying cardiovascular and kidney disease and older patients.
As a consequence of trials resulting from the 2008 guidance, major national and international guidelines now recommend an SGLT2i or GLP-1 RAs in patients with T2DM with established atherosclerotic cardiovascular disease (ASCVD) or high CV risk.7-10 In 2020, the FDA approved dapagliflozin, an SGLT2i, for the treatment of HFrEF in adults with and without T2DM, marking the first time that a drug class developed for diabetes was successfully approved to treat HF, even when diabetes is not present.11 Dapagliflozin is now also approved to reduce the risk of adverse kidney and cardiovascular outcomes in patients with CKD who are at risk of disease progression, irrespective of whether they have diabetes.12
For the first time, clinical trials have successfully shown that a therapy can improve outcomes for patients with heart failure with preserved ejection fraction (HFpEF), which is recognized as a major public health problem with increasing prevalence worldwide. This was first shown in the pooled analysis of SOLOIST-WHF and SCORED data – sotagliflozin, a dual SGLTi, was shown to reduce the risk of CVD death, HF events, and the benefits were preserved irrespective of baseline EF, including HFpEF.13,14 Findings from EMPEROR-Preserved demonstrate the potential of SGLT2i (empagliflozin) to reduce the risk for hospitalization and cardiovascular death for patients with HFpEF. However, despite proven CV benefit and multiple society guideline recommendations, the use of these medicines remains profoundly limited, and strategies to improve their uptake in clinical practice are urgently needed.
These past 12 years of CVOTs paved the way for new classes of glucose lowering drugs with proven CV benefit including the SGLT2i and GLP-1 RAs which have consistently shown reduction in ischemic events in patients with diabetes and ASCVD. Additionally, groundbreaking CVOTs of SGLT2i went on to show significant cardiorenal benefits with risk reduction in HF and kidney disease broadening its use in patients with diabetes. Future trials should continue to maintain the pipeline of rigorous multi-system evaluation of glucose lowering drugs with further innovations in fields like obesity, fatty liver disease, and arrhythmia.
References
- Guidance for Industry Diabetes Mellitus-Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes (FDA website). 2008. Available at: https://www.fda.gov/media/71297/download. Accessed 03/18/2021.
- Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control Guidance for Industry (FDA website). 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/type-2-diabetes-mellitus-evaluating-safety-new-drugs-improving-glycemic-control-guidance-industry. Accessed 07/13/2021.
- McMurray JJV, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014;2:843-51.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413-24.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008.
- Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776-85.
- Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:1117-45.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487-93.
- Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255-323.
- American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes - 2021. Diabetes Care 2021;44:S125-S150.
- FDA approves new treatment for a type of heart failure (FDA website). 2020. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed 07/14/2021.
- FDA Approves Treatment for Chronic Kidney Disease (FDA website). 2021. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease. Accessed 08/08/2021.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117-28.
- Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129-39.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Diabetes and Cardiometabolic Disease
Keywords: Diabetes Mellitus, Type 2, Cardiovascular Diseases, Heart Failure, Sodium-Glucose Transporter 2 Inhibitors, Creatinine, Glucagon-Like Peptide-1 Receptor, Dipeptidyl Peptidase 4, Dipeptidyl-Peptidase IV Inhibitors, Glomerular Filtration Rate, Glucagon-Like Peptide 1, Odds Ratio, Glucose, Pharmaceutical Preparations, Drug Evaluation, United States Food and Drug Administration, Confidence Intervals, Albumins, Public Health, Glycemic Control, Stroke Volume, Renal Dialysis, Risk Factors, Kidney Failure, Chronic, Renal Insufficiency, Chronic, Hospitalization, Myocardial Infarction, Arrhythmias, Cardiac, Risk Reduction Behavior, Stroke, Obesity, Disease Progression, Heart Disease Risk Factors, Liver Diseases
< Back to Listings