From the Starting Line | Left Main Revascularization: Primer For Early Career Cardiologists
A 60-year-old man unvaccinated for COVID-19 presents to an emergency department of a community hospital with severe chest pain and shortness of breath lasting one to two hours. His medical history is significant for hypertension, diabetes, hyperlipidemia and active COVID-19 (on treatment with casirivimab/imdevimab). He is tachycardiac (heart rate 138 bpm), tachypneic (respiratory rate 37 breaths/minute) and slightly hypertensive (blood pressure 139/100 mm Hg). Chest X-ray shows diffuse bilateral airspace disease.
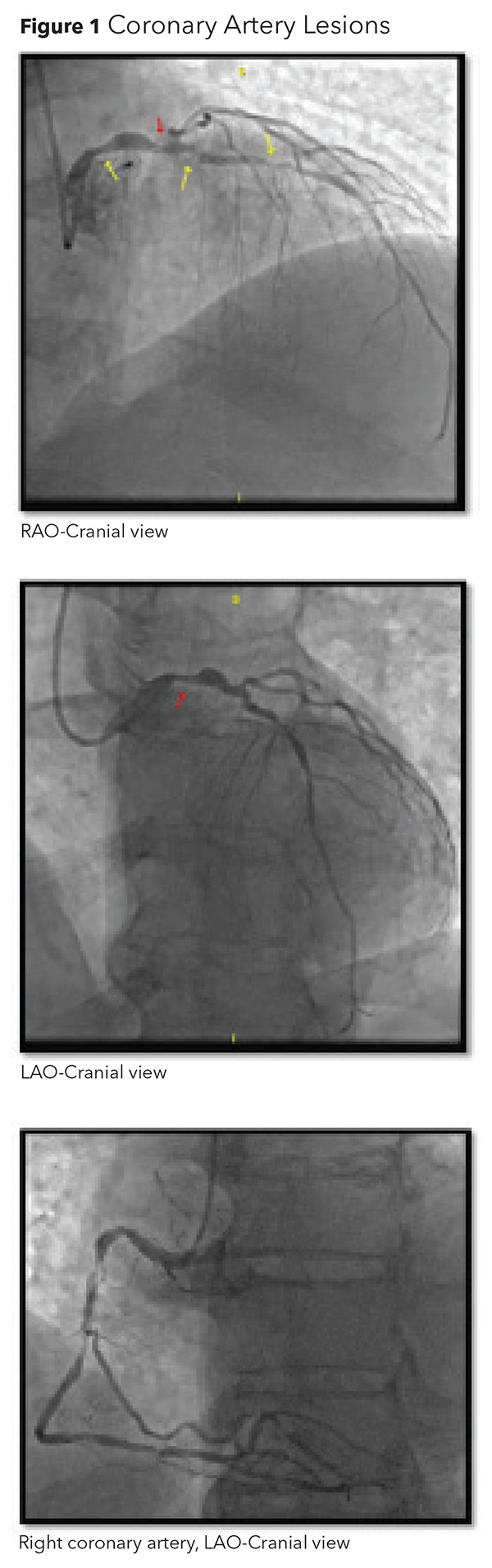
The patient's electrocardiogram shows ST elevation in leads V2 -V6 concerning for an acute anterior STEMI. The cardiac cath lab is activated. Aspirin 324 mg, clopidogrel 600 mg and IV heparin 7000 Unit bolus are administered and bilevel positive airway pressure is initiated for respiratory support. Diagnostic coronary angiography reveals an 80% ostial left main (LM) lesion stenosis, multiple stenoses in the left anterior descending artery (LAD) with TIMI 2 flow distally (80% proximal LAD, 99% mid LAD and 70% distal LAD), an anomalous left circumflex (LCx) which arose from the right cusp and multiple obstructive lesions in the right coronary artery (Figure 1).
Question: What is your revascularization strategy?
- PCI to the infarct-related artery (IRA) only.
- Immediate PCI to the IRA and refer for LM artery surgical revascularization.
- Immediate PCI to the LAD and LM vessel.
- Multivessel PCI to the LAD, LM, LCx and right coronary artery.
- Insert a mechanical circulatory support device and refer for surgical revascularization.
Case Review: Clinical Setting
Primary PCI For IRA vs. Complete Revascularization
In acute STEMI, primary PCI of the IRA is recommended (Class I, ESC 2017 guideline).1 In STEMI patients with multivessel coronary artery disease (CAD), non-IRA revascularization should be considered prior to hospital discharge (Class IIa, Level of Evidence A, ESC 2018 guideline).2
Emergency surgical revascularization can be considered for STEMI patients with IRA anatomy not suitable for PCI, ongoing ischemia and a large myocardial area at risk (Class IIa, ESC 2018 guideline). In patients for whom surgical revascularization is intended, if a large myocardium is at risk or there is hemodynamic instability, the guideline allows for proceeding with surgery after discontinuing dual antiplatelet therapy (DAPT) without the usual waiting period for full recovery of platelet function (ESC 2017 guideline).
Cardiogenic Shock
A 2021 American Heart Association scientific statement recommends PCI of the IRA for acute MI complicated by cardiogenic shock regardless of time delay.3 The scientific statement further recommends that for most patients, PCI should be limited to the culprit lesion with possible staged revascularization of the nonculprit lesions. This approach is supported by the ESC 2018 guideline, which recommends against routine revascularization of the non-IRA lesion during STEMI PCI complicated by cardiogenic shock (Class III).
The AHA scientific statement also supports a hybrid approach of culprit lesion PCI (with or without stent placement) followed by staged surgical revascularization for patients with acute MI complicated by cardiogenic shock and multivessel CAD. Emergency surgical revascularization is recommended for patients with cardiogenic shock if the coronary anatomy is not suitable for PCI (Class I, ESC 2018 guideline).
COVID-19 Positive Patients
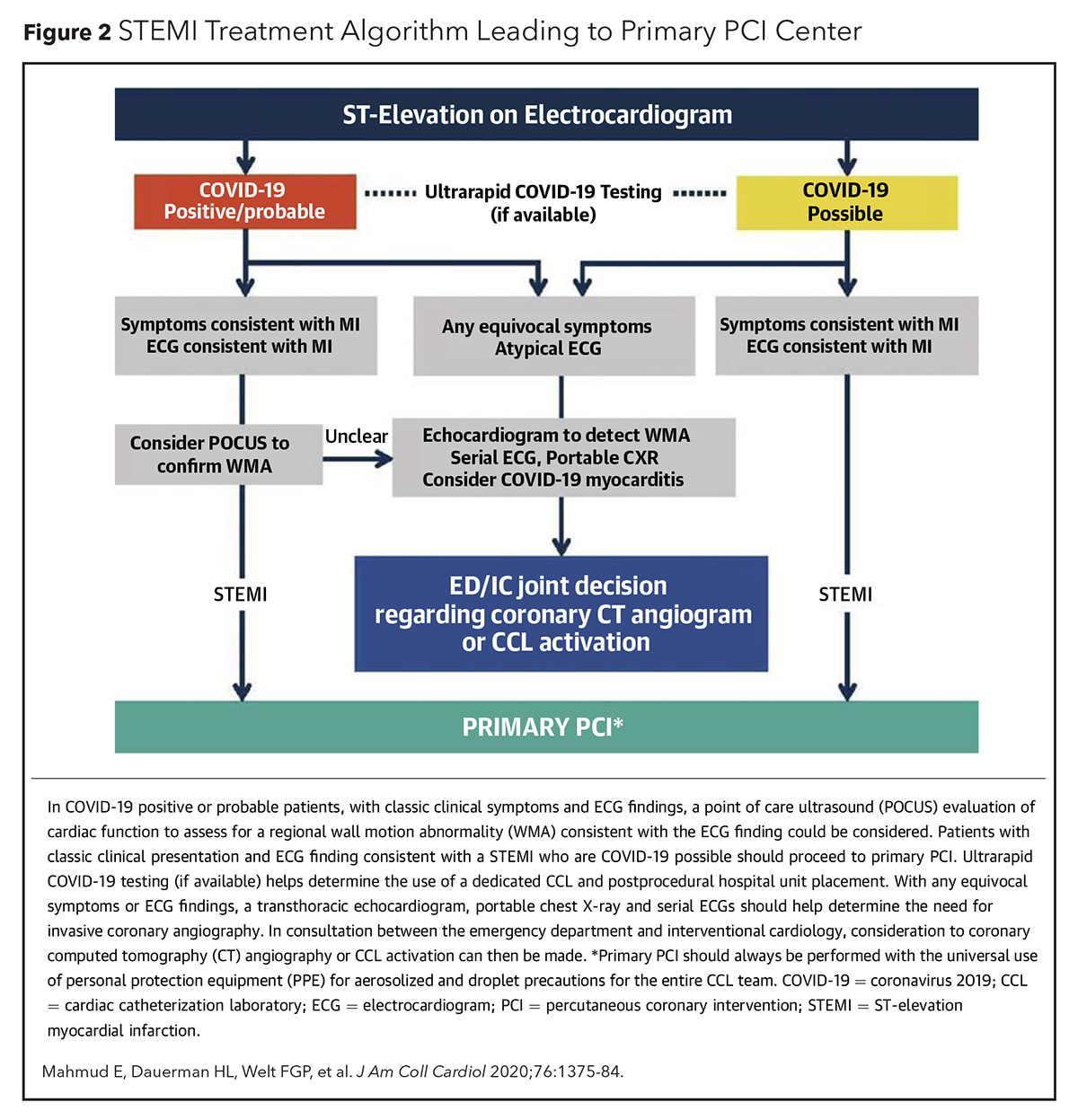
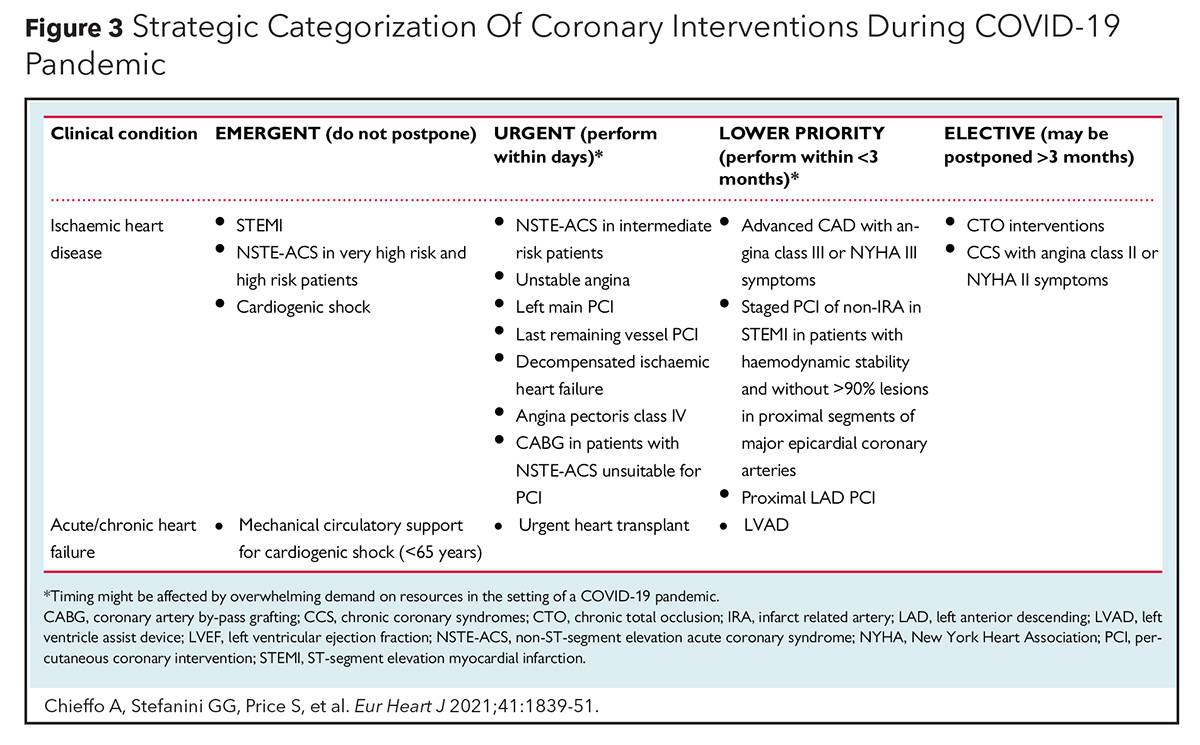
Primary PCI as the main reperfusion strategy for STEMI has been recommended by several cardiovascular societies. ACC, SCAI and the American College of Emergency Physicians recommend PCI as the main/preferred reperfusion strategy for STEMI patients during the COVID-19 pandemic (Figure 2).4 Primary PCI as first-line therapy for STEMI is also recommended by the European Association of Percutaneous Cardiovascular Intervention (EAPCI) if it can be performed within 120 minutes from symptom onset (Figure 3).5 The North American Society Leadership also recommends primary PCI for most STEMI patients and selective pharmacoinvasive therapy in accordance with regional practice.6,7
Regarding the location for the management of STEMI patients with cardiogenic shock who are positive for COVID-19, the EAPCI recommends expert centers which can provide different options for mechanical circulatory support.
For COVID-19-positive patients experiencing a STEMI with mechanical complications, an intra-aortic balloon pump (IABP) is an option if other mechanical circulatory support devices are not available. Veno-arterial extracorporeal membrane oxygenation (VA ECMO) is the recommended device for COVID-19 patients with hemodynamic and respiratory failure. Impella or IABP can be used for management of left ventricular overdistension in VA ECMO patients.
Initial data from the North American COVID-19 Myocardial Infarction Registry reveals that primary PCI is the predominant reperfusion strategy for COVID-19-positive STEMI patients.8 It will be interesting and valuable to see the long-term patient outcomes data from this registry.
Procedural Characteristics
Left Main Artery Stenosis
Given the recommendations regarding a non-IRA, consideration should be made regarding the best revascularization strategy for an unprotected LM artery stenosis detected during an anterior STEMI. Significant LM disease, defined as ≥50% stenosis, is associated with increased morbidity and a five-year mortality rate of 57%, when not revascularized due to the large myocardial area at risk.9
Revascularization Trials of LM Disease
Three large randomized clinical trials have compared PCI to CABG for LM artery disease. The SYNTAX trial randomized 1,800 patients to PCI with first-generation TAXUS drug-eluting stents (DES) or CABG.10 Patients were divided into three tertiles based on lesion complexity (SYNTAX scores 0-22, 23-32 and >32). The LM subgroup comprised 705 patients. For patients with complex disease (SYNTAX score >32), CABG was superior to PCI with lower rates of major adverse cardiovascular and cerebrovascular events (MACCE). The benefit was primarily driven by a significant decrease in repeat revascularization rates. In the other two tertiles, MACCE outcomes were similar for CABG and PCI.
NOBLE, a noninferiority trial, randomized 1,201 patients with LM disease (mean SYNTAX score 23) to either PCI with a biolimus-eluting stent (BioMatrix Flex) or CABG.11 At the five-year follow-up, CABG performed better than PCI, with a significantly lower rate of MACCE (all-cause mortality, nonprocedural MI, repeat revascularization or stroke): 19% vs. 28% in the as-treated analysis. No difference was found between CABG and PCI, respectively, for all-cause mortality (9% vs. 12%; p=0.0040) and stroke (2% vs. 5%; p=0.073).
EXCEL, also a noninferiority trial, randomized 1,905 patients with LM stenosis (low or intermediate SYNTAX scores <32) to PCI with the second-generation everolimus (Xience) DES or CABG.12 The landmark analysis for the period of 30 days to three years showed a significant difference for the primary endpoint in favor of CABG (7.9 vs. 11.5%; p=0.02). The primary outcome showed noninferiority (no between-group difference) for the composite of death from any cause, stroke or MI at three years (15.4% PCI vs. 14.7% CABG; hazard ratio [HR], 1.0; 95% confidence interval [CI], 0.79-1.26; p=0.98 for superiority). At five years, no significant difference was seen between PCI and CABG for the composite outcome of death, stroke or MI. The study investigators concluded the EXCEL trial data do not a priori support a preferential role for PCI over CABG in patients with LM disease and known prior cerebrovascular disease due to a higher rate of stroke seen in this patient subgroup.13
A meta-analysis of four clinical trials comparing PCI vs. CABG for LM stenosis (EXCEL, NOBLE, PRECOMBAT, SYNTAX) showed comparable outcomes for both revascularization strategies in patients with low-to-intermediate complexity, but more repeat revascularization after PCI (HR, 1.70; 95% CI, 1.42-2.05; p<0.001).14 Another meta-analysis of five clinical trials (EXCEL, NOBLE, PRECOMBAT, SYNTAX, LEMANS) comparing PCI against CABG for LM stenosis showed no significant difference in the composite endpoint of death, stroke and MI (odds ratio [OR], 1.03, 95% CI, 0.80-1.4; p=0.70) and no difference in all-cause death (OR, 1.03; 95% CI, 0.81-1.32; p=0.81). However, a significantly higher rate of repeat revascularization was seen in the PCI group (OR, 1.76; 95% CI, 1.45-2.13; p<0.001).15
Guideline Recommendations For LM Revascularization
The ESC guideline recommends CABG for LM stenosis for all SYNTAX score groups (Class I, ESC 2018 guideline). However, PCI for LM stenosis is recommended for patients with a low SYNTAX score <22 (Class I, ESC 2018 guideline) and PCI for LM stenosis in patients with an intermediate SYNTAX score can be performed (Class IIa, ESC 2018 guideline).
Three documents recommend against PCI for LM stenosis in patients with a high SYNTAX score (≥33), including the ACC 2011 guideline,16 the ACC/AHA 2016 appropriate use criteria for coronary revascularization in patients with acute coronary syndromes17 and the 2018 ESC guideline (Class III). The 2011 ACC guideline recommends PCI for LM stenosis (Class IIa for low SYNTAX score and Class IIb for intermediate SYNTAX score).
Procedural Considerations
- DES are recommended.
- SYNTAX score should be calculated. In the EXCEL trial, the STS risk score was shown to have poor discrimination for mortality (C statistic 0.507), but good discrimination for stroke (C statistic 0.751).18 The predictive performance of the STS score for renal failure was good for CABG (C statistic 0.82), but poor for PCI (C statistic 0.59).
- Outcomes differ by LM lesion location. Ostial or LM shaft PCI is associated with lower risk for repeat revascularization vs. distal LM bifurcation.19 At three years, EXCEL showed that ischemia-driven revascularization occurred more frequently after PCI than CABG in patients with LM distal bifurcation disease (13.0% vs. 7.2%; OR, 2.00; 95% CI, 1.41-2.85; p=0.0001), but was not significantly different in patients with disease only at the LM ostium or shaft (9.7% vs. 8.4%; OR, 1.18; 95% CI, 0.52-2.69; p=0.68) (p interaction, 0.25).20 PCI for distal LM bifurcation disease is associated with worse long-term outcomes vs. CABG.21
- PCI technique affects outcomes. The DK crush technique, vs. provisional stenting for LM distal bifurcation lesions, at one year was associated with lower rates of target lesion failure (5% vs. 10.7%; p=0.02) and stent thrombosis (0.4% vs. 3.3%; p=0.02) in the randomized DKCRUSH-V trial.22 Disease involving the ostial LCx should guide the choice of single-stent or double-stent technique, as it is not uncommon for the ostial Cx to become compromised after LM stent crossover from the proximal LAD to the LM.23 The compromise in the ostial Cx occurs predominantly from carina shift.
- IVUS helps. Distal LM bifurcation PCI can be optimized by using IVUS to ensure optimal stent expansion at both the ostial LAD, ostial LCx and the distal LM.24 Angiographic in-stent restenosis is predicted with IVUS-minimal stent area of 8.2 mm2 for the proximal LM above the polygon of confluence, 7.2 mm2 for the polygon of confluence, 6.3 mm2 for the LAD artery ostium and 5.0 mm2 for the LCx ostium. IVUS is useful for ostial LM stent positioning.
Case Wrap-Up
Question Recap
What is your revascularization strategy?
It should be immediate PCI to the LAD and LM vessel.
An IABP was inserted for hemodynamic support because the patient had SCAI Stage B shock. His SYNTAX score was 37. In keeping with our team-based care approach, we reviewed the patient's imaging with cardiovascular surgery and a joint decision was made to proceed with PCI. Using IV tirofiban bolus and infusion drip, we performed culprit-vessel revascularization, including treatment of LM disease, because of the volume of the myocardium at risk.
DES were deployed in the distal, mid and proximal LAD (2.5 x 38 mm, 3.0 x 32 mm, 3.0 x 24 mm, respectively). The ostial LM artery was treated with a 4.0 x 20 mm DES, which was postdilated with a 4.5 noncompliant balloon. Postprocedure angiography showed TIMI 3 flow in the distal vessel.
The patient was admitted to the intensive care unit. Transthoracic echocardiogram revealed a left ventricular ejection function of 24% with regional wall motion abnormalities and no hemodynamically significant valvular disease. DAPT with aspirin and clopidogrel was initiated, along with atorvastatin, lisinopril and spironolactone. The IABP was successfully removed after 24 hours and he was weaned off supplemental oxygen. Our infectious disease colleague recommended a five-day course of remdesivir and a 10-day course of dexamethasone for treatment of COVID-19 pneumonitis. Our patient was successfully discharged home on Day 6 to complete a two-week period of self-isolation for COVID-19 infection.
Take-Home Points
- A Heart Team approach, with shared-decision-making with the patient, is recommended when considering revascularization options.
- Primary PCI is recommended for COVID-19 patients with STEMI.
- Culprit-only (IRA) revascularization is recommended for STEMI patients.
- Emergent surgical revascularization for COVID-19-positive STEMI patients with acute COVID pneumonitis/pneumonia is most likely not feasible at most health care centers.
- There is equipoise between percutaneous and surgical revascularization strategies for LM stenosis with low-to-intermediate SYNTAX score.
- Procedural management of ostial and mid shaft LM disease differs from distal LM disease.

Nkechi Ijioma, MD, FACC, is senior associate consultant cardiologist with the Mayo Clinic Health System in La Crosse, WI. She acknowledges and appreciates the expert content review by Jose Emilio Exaire, MD, FACC, at Mayo Clinic; Kwan S. Lee, MD, FACC, at the University of Arizona, Tucson; and S. Michael Gharacholou, MD, at Mayo Clinic.
References
- Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology. Eur Heart J 2018;39:119-77.
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165.
- Henry TD, Tomey MI, Tamis-Holland JE, et al. Invasive management of acute myocardial infarction complicated by cardiogenic shock: A scientific statement from the American Heart Association. Circulation 2021;143:e815-e829.
- Mahmud E, Dauerman HL, Welt FGP, et al. Management of acute myocardial infarction during the COVID-19 pandemic: A position statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol 2020;76:1375-84.
- Chieffo A, Stefanini GG, Price S, et al. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur Heart J 2021;41:1839-51.
- Wood DA, Mahmud E, Thourani VH, et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: From the North American Society Leadership. J Am Coll Cardiol 2020;75:3177-83.
- Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic. From ACC's Interventional Council and SCAI. J Am Coll Cardiol 2020;75:2372-5.
- Garcia S, Dehghani P. Grines C, et al. Initial findings from the North American COVID-19 myocardial infarction registry. J Am Coll Cardiol 2021;77:1994-2003.
- Bruschke AV, Proudfit WL, Sones FM Jr. Progress study of 590 consecutive nonsurgical cases of coronary disease followed 5-9 years. II. Ventriculographic and other correlations. Circulation 1973;47:1154-63.
- Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70.
- Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-52.
- Stone GW, Sabik JF, Serruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–35.
- Diamond J, Madhavan MV, Sabik JF 3rd, et al. Left main percutaneous coronary intervention versus coronary artery bypass grafting in patients with prior cerebrovascular disease: Results from the EXCEL trial. JACC Cardiovasc Interv 2018;11:2441-50.
- Giacoppo D, Colleran R, Cassese S, et al. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis: A systematic review and meta-analysis. JAMA Cardiol 2017;2:1079-88.
- De Rosa S, Polimeni A, Sabatino J, Indolfi C. Long-term outcomes of coronary artery bypass grafting versus stent-PCI for unprotected left main disease: a meta-analysis. BMC Cardiovasc Disord 2017;17:240.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J Am Coll Cardiol 2011;58:e44-122.
- Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ ASNC/SCAI/SCCT/ STS 2016 appropriate use criteria for coronary revascularization in patients with acute coronary syndromes. J Am Coll Cardiol 2017;69:570-91.
- Thuijs DJFM, Habib RH, Head SJ, et al. Prognostic performance of the Society of Thoracic Surgeons risk score in patients with left main coronary artery disease undergoing revascularisation: a post hoc analysis of the EXCEL trial. EuroIntervention 2020;16:36-43.
- Naganuma T, Chieffo A, Meliga E, et al. Long-term clinical outcomes after percutaneous coronary intervention for ostial/mid-shaft lesions versus distal bifurcation lesions in unprotected left main coronary artery: the DELTA Registry (drug-eluting stent for left main coronary artery disease): a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc Interv 2013;6:1242-49.
- Gershlick AH, Kandzari DE, Banning A, et al. Outcomes after left main percutaneous coronary intervention versus coronary artery bypass grafting according to lesion site: Results from the EXCEL trial. JACC Cardiovasc Interv 2018;11:1224-33.
- Hyun J, Kim JH, Jeong Y; MAIN-COMPARE Registry. Long-term outcomes after PCI or CABG for left main coronary artery disease according to lesion location. JACC Cardiovasc Interv 2020;13:2825-36.
- Chen S-L, Zhang J-J, Han Y, et al. Double kissing crush versus provisional stenting for left main distal bifurcation lesions. J Am Coll Cardiol 2017;70:2605-17.
- Kang SJ, Mintz GS, Kim WJ, et al. Changes in left main bifurcation geometry after a single-stent crossover technique: an intravascular ultrasound study using direct imaging of both the left anterior descending and the left circumflex coronary arteries before and after intervention. Circ Cardiovasc Interv 2011;4:355-361.
- Kang SJ, Ahn JM, Song H, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv 2011;4:562-569.
Clinical Topics: Anticoagulation Management, Cardiac Surgery, Cardiovascular Care Team, COVID-19 Hub, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Stable Ischemic Heart Disease, Valvular Heart Disease, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Aortic Surgery, Cardiac Surgery and Heart Failure, Cardiac Surgery and SIHD, Cardiac Surgery and VHD, Acute Heart Failure, Interventions and Coronary Artery Disease, Interventions and Imaging, Interventions and Structural Heart Disease, Interventions and Vascular Medicine, Angiography, Nuclear Imaging, Hypertension, Chronic Angina
Keywords: ACC Publications, Cardiology Magazine, American Heart Association, Angiography, Arteries, Aspirin, Blood Pressure, Communicable Diseases, Confidence Intervals, Constriction, Pathologic, Coronary Angiography, Coronary Artery Bypass, Coronary Artery Disease, Coronary Restenosis, COVID-19, Decision Making, Shared, Delivery of Health Care, Dexamethasone, Diabetes Mellitus, Drug-Eluting Stents, Electrocardiography, Emergency Service, Hospital, Everolimus, Extracorporeal Membrane Oxygenation, Family Characteristics, Follow-Up Studies, Heart Rate, Heart Valve Diseases, Hemodynamics, Heparin, Hospitals, Community, Hypertension, Intensive Care Units, Lisinopril, Middle Aged, Morbidity, Myocardial Infarction, Myocardium, Odds Ratio, Oxygen, Patient Discharge, Percutaneous Coronary Intervention, Platelet Aggregation Inhibitors, Registries, remdesivir, Renal Insufficiency, Reperfusion, Respiratory Insufficiency, Respiratory Rate, Risk Factors, Shock, Cardiogenic, Spironolactone, ST Elevation Myocardial Infarction, Stents, Stroke, Taxus, Thrombosis, X-Rays
< Back to Listings