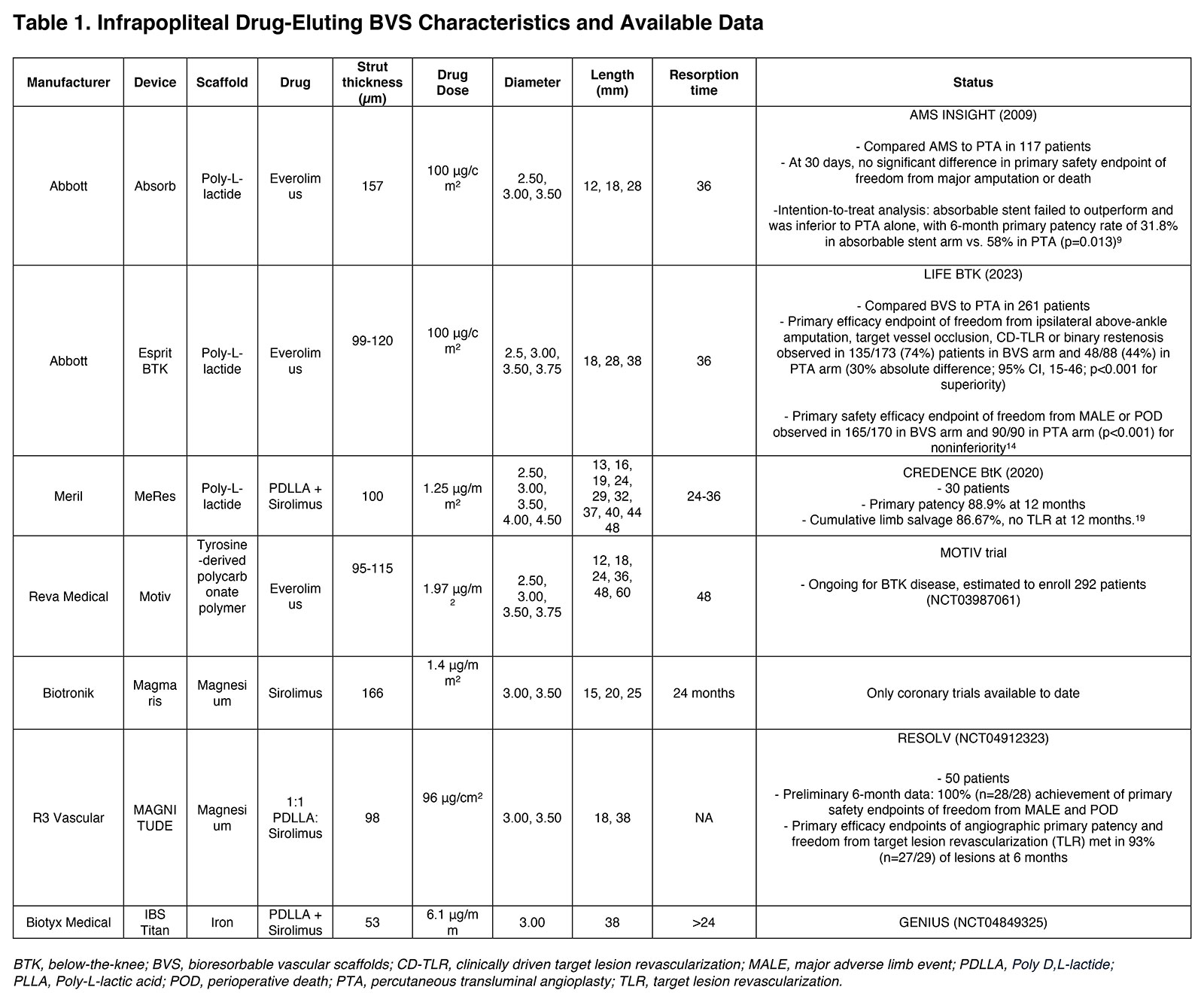
Peripheral Matters | Bioresorbable Stents in Below-the-Knee Arterial Disease

Below-the-knee (BTK) arterial disease represents a significant challenge in peripheral artery disease (PAD) management, particularly in patients with chronic limb-threatening ischemia (CLTI).
CLTI characterized by ischemic rest pain or tissue loss of greater than two weeks duration attributable to PAD affects a small but significant portion of PAD patients. With an annual incidence of 2.3% and an estimated mortality of 20-25% at one year, patients with CLTI face the gravest prognosis.1-3
Over 70% of patients with diabetes and CLTI have infrapopliteal or BTK disease with a class I recommendation for revascularization to improve wound healing.4
Despite the increasing frequency of endovascular intervention, management of BTK lesions remain challenging owing to the smaller caliber of the vessel, frequent comorbidities such as diabetes and end-stage renal disease, and the propensity for long, diffuse and calcified lesions.
However, the unmet clinical need for a device which can resist vascular recoil, treat flow-limiting dissection and deliver an antiproliferative drug to combat restenosis has led to the development of a variety of drug-eluting bioresorbable scaffolds (DRS).
Rationale For Bioresorbable Stents

Bioresorbable stents (BRS) were initially developed with the goal of providing adequate mechanical support and drug delivery in the short to medium term (one to two years) while gradually disappearing over time. This approach aligns with the popular "leave nothing behind" strategy in endovascular interventions.
Long-term patency of BTK PAD lesions is limited by three main factors: elastic recoil of the target vessel, flow-limiting dissection and restenosis due to neointimal hyperplasia.
BRS can preserve native vessel anatomy and function, maintain natural vasomotion crucial for adequate tissue perfusion, allow for potential future interventions and preserve options for surgical bypass without the complications associated with permanent implants, and eliminate artifacts during noninvasive imaging procedures.
In patients with dissections, perforations that could not be conservatively managed, for which coronary drug-eluting stents (DES) or sacrificing the artery by embolization were the only options, now DRS can be used.
Simultaneous drug elution delivers antiproliferative agents to vessels post percutaneous transluminal angioplasty (PTA) to prevent the inflammatory cascade leading to long-term restenosis.
Composition of Bioresorbable Stents
The composition of drug-eluting BRS has evolved significantly since their inception, with various materials being explored to achieve the optimal balance of mechanical properties, biocompatibility and degradation profiles. DRS for BTK interventions include scaffolds and the drug. The scaffolds can be made of polymers and corrodible metals.
Polymers
Figure 1: Esprit™ BTK Everolimus Eluting Bioresorbable Scaffold System. Used with permission from Abbott.

Poly-L-lactic acid (PLLA) has been the most widely used polymer in bioresorbable scaffolds. It's a semi-crystalline polymer that degrades through hydrolysis of its ester linkages. The degradation products, primarily lactic acid, enter the Krebs cycle and are eventually eliminated as carbon dioxide and water.
Advantages of PLLA include its biocompatibility, controllable degradation rate and ability to be processed into high-strength fibers. However, PLLA-based stents have limitations such as lower radial strength compared with metallic stents, requiring thicker struts.
The Absorb bioresorbable vascular scaffold (BVS; Abbott Vascular), ESPRIT BRS (Abbott Vascular) and MeRes (Meril) are primarily composed of PLLA. The initial Absorb stent was a 157 μm-thick scaffold and a poly-D, L-lactic acid (PDLLA) coating containing everolimus.
The novel Esprit BTK scaffold represents a significant advancement over the Absorb stent with lower strut thickness of 99 µm to 120 µm. It facilitates the controlled release of everolimus at a concentration of 100 µg/mm2, matching that of the coronary Xience DES.5
Figure 2: First Generation Absorb Bioresorbable Vascular Scaffold. Representative photomicrographs of porcine coronary arteries implanted with Absorb bioresorbable vascular scaffold. Degrading polymer is replaced by a cellular matrix.

The MAGNITUDE DRS (R3 Vascular) is a sirolimus-eluting BVS composed of a PLLA polymer matrix with a PDLLA coating. It features a 98 µm strut thickness, high radial strength and lengths up to 58 mm.6
Other polymers being studied include Poly(D,L-lactide-co-glycolide) (PLGA) and tyrosine-derived polycarbonate (Tyrocore). Tyrocore material, used in the MOTIV BVS (Reva Medical), has a strut thickness of 95 μm to 115 μm and is designed to have improved mechanical properties and a more controlled degradation profile compared with PLLA.
Tyrocore-based stents can have thinner struts while maintaining adequate radial strength, potentially improving deliverability in small BTK vessels and patients are currently being enrolled in a pivotal IDE trial in the U.S.7
Corrodible Metal Stents
Metallic BRS represent an innovative approach in vascular intervention, aiming to provide temporary support to treated vessels while eventually being fully resorbed by the body. This technology seeks to address limitations of both permanent metallic stents and polymeric bioresorbable scaffolds.
Magnesium alloys have been at the forefront of this development, with the AMS-1 stent pioneering the field using a WE43 alloy. Magnesium offers several advantages, including biocompatibility, higher radial strength compared with polymers, and well-tolerated degradation products.
However, early iterations faced challenges with rapid corrosion. Newer generations, such as the Magmaris stent (Biotronik), incorporate elements like yttrium and zirconium to slow the degradation process, providing mechanical support for several.
Iron-based alloys have also been investigated for BRS, offering high radial strength and slower degradation compared with magnesium. Their high ductility enhances deliverability through catheter-based systems, making them particularly suitable for calcified and occluded vessels and degraded iron can inhibit proliferation of vascular smooth muscle cells, which can decrease in-stent restenosis.
However, concerns about the long-term effects of iron oxide accumulation in tissues have limited their clinical application thus far. IBS Titan is an iron-based scaffold, with lengths up to 118 mm designed to treat long segment BTK lesions.8
More recently, zinc-based alloys have emerged as a promising material for BRS. These alloys potentially offer an optimal balance between support duration and complete resorption, with a degradation rate between that of magnesium and iron. However, research on zinc-based stents is still in early stages, primarily focused on preclinical studies.
Drug Delivery
The DRS incorporate antiproliferative agents, primarily rapamycin derivatives and paclitaxel, to inhibit intimal hyperplasia and smooth muscle cell proliferation. The drug release kinetics are mediated by the biodegradation of an amorphous polymer matrix coating the polymeric or metallic backbone. This matrix undergoes hydrolysis over months, facilitating controlled drug elution, while the scaffold degrades over one to three years.
Clinical Studies and Outcomes

The clinical journey of BRS in BTK interventions began in 2005 with the study of the absorbable metal stent (AMS). This initial investigation showed promising results, with one-year primary patency, limb salvage and survival rates of 73.3%, 94.7% and 85%, respectively.
However, the subsequent AMS INSIGHT trial, comparing AMS to PTA in 117 patients, revealed challenges. While there was no difference in 30-day safety endpoints, AMS showed inferior six-month patency compared with PTA (31.8% vs. 58%; p=0.013).9
A notable study by Varcoe, et al., in 2016 demonstrated encouraging outcomes in 33 patients with Rutherford Class 3-5 symptoms.10 The ABSORB BVS achieved 12-month survival of 84.8%; freedom from clinically-driven target lesion revascularization (CD-TLR) of 96%; and primary patency rates of 96%, 96% and 84.6% at 6, 12 and 24 months, respectively. Long-term follow-up at five years continued to show positive results, and additional retrospective studies have corroborated these findings.11
A meta-analysis of studies with 12-month data reported impressive pooled outcomes: 90% primary patency, 96% freedom from CD-TLR, 97% limb salvage rate and 90% survival at one year.12 A subsequent pooled analysis examining 121 patients who received 189 ABSORB BVS yielded similar results.13
The LIFE-BTK trial was a prospective, multicenter, randomized, controlled study evaluating the efficacy and safety of the Esprit bioresorbable drug-eluting vascular scaffold compared with standard PTA in BTK PAD. The trial enrolled 261 patients with critical limb ischemia (Rutherford categories 4-5) and randomized them in a 2:1 ratio to receive either the Esprit scaffold (n=173) or PTA (n=88).
The primary efficacy endpoint of LIFE-BTK was a composite of freedom from above-ankle amputation of the target limb, target vessel occlusion, CD-TLR or binary restenosis at 12 months. Results demonstrated that 74% of patients in the scaffold group achieved the primary endpoint vs. 44% in the PTA group (absolute difference 30%; 95% CI, 15%-46%; p<0.001).
The primary safety endpoint, defined as freedom from major adverse limb events (MALE) and perioperative death (POD) at six months, was met with noninferiority in the scaffold arm. With a number needed to treat of 4 (95% CI, 2.2-6.7), the ESPRIT trial potentially represents a paradigm shift in the endovascular management of critical limb ischemia.14
The MOTIV BVS BTK pilot study was a prospective, single-arm, multicenter trial evaluating 76 MOTIV scaffolds in 60 limbs over 36 months. The study included lesions with a mean length of 29.46 mm, encompassing both primary de novo and restenotic lesions (n=37) as well as post-PTA complications (n=23). Preliminary six-month data demonstrated 99% technical success, 90% primary patency and a 3% CD-TLR rate.
A subsequent global randomized trial is assessing primary efficacy endpoints of freedom from above-ankle amputation, CD-TLR and target lesion occlusion at six months, with a primary safety endpoint of freedom from all-cause POD and MALE of the index limb involving infrapopliteal arteries at 30 days.15
The RESOLV I trial was a prospective, single-arm, multicenter, first-in-human study evaluating the Magnitude DRS bioresorbable scaffold in infrapopliteal interventions. The study enrolled up to 50 patients with Rutherford-Becker category 3-5 disease, treating lesions with a mean length of 34.6 ± 15.4 mm.
Preliminary six-month data demonstrated 100% (n=28/28) achievement of primary safety endpoints, defined as freedom from MALE and POD. Primary efficacy endpoints, including angiographic primary patency and freedom from target lesion revascularization, were met in 93% (n=27/29) of lesions at six months.
Technical Aspects of Deploying BRS
Vessel preparation for BRS deployment in BTK disease is a critical multistep process aimed at optimizing lesion compliance, decreasing recoil and obtaining maximal luminal gain. Following guidewire crossing, the lesion should be adequately prepped, as needed with either balloons, lithotripsy or atherectomy.
The extent of preparation is tailored to lesion complexity and vessel characteristics. This meticulous approach is particularly crucial for BRS due to their lower radial strength compared with metallic stents.
Subsequent reassessment, often using IVUS or OCT, is crucial to confirm adequate preparation. IVUS has demonstrated superior accuracy in vessel sizing (ranging from 0.5 mm to 1.1 mm) compared with angiography for BTK interventions, with significant implications for treatment outcomes including earlier wound healing.16-18
These findings collectively underscore the potential of IVUS to enhance the accuracy of vessel sizing in BTK interventions, leading to more appropriate device selection, and improved clinical outcomes in patients with CLTI.
Challenges and Future Directions

Despite the promising results of BRS in BTK disease, several significant challenges remain stemming from the inherent properties of the materials used and the unique demands of the BTK vascular bed.
The lower radial strength of BRS compared with metallic stents, particularly in the context of often heavily calcified BTK lesions can potentially lead to higher rates of acute recoil and long-term restenosis. The radial strength of BRS decreases over time as the scaffold begins to degrade, while metallic drug-eluting scaffolds maintain their strength indefinitely.
The LIFE-BTK trial demonstrated superior efficacy of the Esprit scaffold compared with balloon angioplasty in BTK lesions, suggesting that its radial strength is sufficient for its intended use. Two-year data from this trial are to be released soon which may allay or corroborate fears about loss of radial strength over time.
Current BRS are available in a limited range of sizes and lengths, with most ranging from 12 mm to 38 mm. BTK lesions often require treatment of segments >100 mm. This often necessitates the use of multiple overlapping scaffolds, which can increase the risk of restenosis at overlap sites and may impact the degradation profile of the devices. Future scaffold designs with longer scaffolds may overcome this concern.
Determining the optimal duration of scaffold support remains a challenge. Current bioresorbable scaffolds typically provide mechanical support for three to six months before significant degradation occurs. However, it's unclear whether this timeframe is optimal for BTK lesions, which may require longer-term support due to the often diffuse and calcified nature of the disease.
The ideal composition for a BRS in BTK disease remains an active area of research. The goal is to achieve a balance of adequate radial support, controlled drug release and complete resorption, all while maintaining a low profile suitable for small-vessel interventions.
BRS for BTK PAD show promise to provide a balance between the need for immediate scaffolding and long-term vessel health, potentially offering a paradigm shift from permanent vessel caging to temporary scaffolding with restoration of native vessel architecture and function.
As our understanding of vascular biology and materials science advances, we can expect further refinements in the composition of BRS, potentially leading to improved outcomes in the challenging realm of BTK interventions.
This article was authored by Sonal Pruthi, MD; Daniel J. Snyder, MD; Robert S. Zilinyi, MD; Ari J. Mintz, DO; Sanjum S. Sethi, MD, MPH, FACC; and Sahil A. Parikh, MD, FACC, all from the Division of Cardiology, Columbia University Irving Medical Center in New York.
References
- Fereydooni A, Gorecka J, Dardik A. Using the epidemiology of critical limb ischemia to estimate the number of patients amenable to endovascular therapy. Vasc Med 2020;25:78-87.
- Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol 2020;40:1808-17.
- Kwong M, Rajasekar G, Utter GH, et al. Updated estimates for the burden of chronic limb-threatening ischemia in the Medicare population. J Vasc Surg 2023;77:1760-75.
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive Summary. J Am Coll Cardiol 2017;69:1465-1508.
- Kim TI, Schneider PA. New innovations and devices in the management of chronic limb-threatening ischemia. J Endovasc Ther 2020;27:524-39.
- Study of the R3 Vascular Drug-Eluting Bioresorbable Scaffold in Treating Below the Knee Arterial Disease (RESOLV I). NCT04912323. Available here.
- MOTIV BTK Randomized Controlled Trial. NCT03987061. Available here.
- IBS Titan™ Sirolimus-eluting Iron Bioresorbable Peripheral Scaffold System Clinical Trial. NCT05971394. Available here.
- Bosiers M, Peeters P, D'Archambeau O, et al. AMS INSIGHT--absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc Intervent Radiol 2009;32:424-35.
- Varcoe RL, Schouten O, Thomas SD, Lennox AF. Experience with the absorb everolimus-eluting bioresorbable vascular scaffold in arteries below the knee: 12-month clinical and imaging outcomes. JACC Cardiovasc Interv 2016;9:1721-28.
- Varcoe RL, Menting TP, Thomas SD, Lennox AF. Long-term results of a prospective, single-arm evaluation of everolimus-eluting bioresorbable vascular scaffolds in infrapopliteal arteries. Catheter Cardiovasc Interv 2021;97:142-49.
- Ipema J, Kum S, Huizing E, et al. A systematic review and meta-analysis of bioresorbable vascular scaffolds for below-the-knee arterial disease. Int Angiol 2021;40:42-51.
- Huizing E, Kum S, Ipema J, et al. Mid-term outcomes of an everolimus-eluting bioresorbable vascular scaffold in patients with below-the-knee arterial disease: A pooled analysis of individual patient data. Vasc Med 2021;26:195-99.
- Varcoe RL, DeRubertis BG, Kolluri R, et al. Drug-eluting resorbable scaffold versus angioplasty for infrapopliteal artery disease. N Engl J Med 2024;390:9-19.
- Lim E, Varcoe RL. Current status of and future prospects for drug-eluting stents and scaffolds in infrapopliteal arteries. J Clin Med 2024;13.
- Shammas NW, Shammas WJ, Jones-Miller S, et al. Optimal vessel sizing and understanding dissections in infrapopliteal interventions: Data from the iDissection Below the Knee Study. J Endovasc Ther 2020;27:575-80.
- Soga Y, Takahara M, Ito N, et al. Clinical impact of intravascular ultrasound-guided balloon angioplasty in patients with chronic limb threatening ischemia for isolated infrapopliteal lesion. Catheter Cardiovasc Interv 2021;97:E376-E384.
- Fujihara M, Yazu Y, Takahara M. Intravascular ultrasound-guided interventions for below-the-knee disease in patients with chronic limb-threatening ischemia. J Endovasc Ther 2020;27:565-574.
- Someshwar V, Thakore V, Banode P, et al. TCT CONNECT-317 Twelve-month clinical outcomes of sirolimus-eluting bioresorbable peripheral scaffold system following percutaneous transluminal angioplasty of below-the-knee arteries in patients with critical limb ischemia: The CREDENCE BtK-1 Study. J Am Coll Cardiol 2020;76 (17 Supplement S):B136-B137.
Clinical Topics: Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Interventions and Vascular Medicine
Keywords: Cardiology Magazine, ACC Publications, Absorbable Implants, Peripheral Arterial Disease, Hyperplasia, Ischemia, Angioplasty, Stents