Focus on EP | The Next Frontier of Pulsed Field Ablation: Ventricular Arrhythmias?

When pulsed field ablation (PFA) achieved European CE mark approval in January 2021 and U. S. Food and Drug Administration (FDA) approval in December 2023, it opened up the proverbial Pandora's Box for treating not only atrial fibrillation (AFib), but also ventricular arrhythmias.
Despite the intended use of PFA for pulmonary vein isolation (PVI), many cardiac electrophysiologists (EPs) are now comfortable performing PFA to address atrial substrates beyond PVI, including the posterior wall, the cavotricuspid and mitral isthmus, and even focal targets. Not surprisingly, an even bolder subset of EPs has begun experimenting with PFA in the ventricles.
The same safety and efficacy profile of PFA in the atrium also make it desirable for use in the ventricle. Unlike thermal ablation modalities, PFA is less likely to cause contact-related tissue trauma (i.e., ectopy and cardiac perforation) as only proximity to the target is required. It's also less likely to result in coagulum formation and thrombus-related cardioembolic complications, and less likely to result in scar contraction and subsequent reduction in myocardial contraction.
Furthermore, the PFA pulse characteristics could be further optimized to limit injury to nearby nervous, connective and vascular tissue. Compared to thermal ablation modalities, PFA lesion application is faster and newer generation PFA catheters have an adjustable distal tip configuration allowing for both focal and larger footprint ablation (Figure 1).1
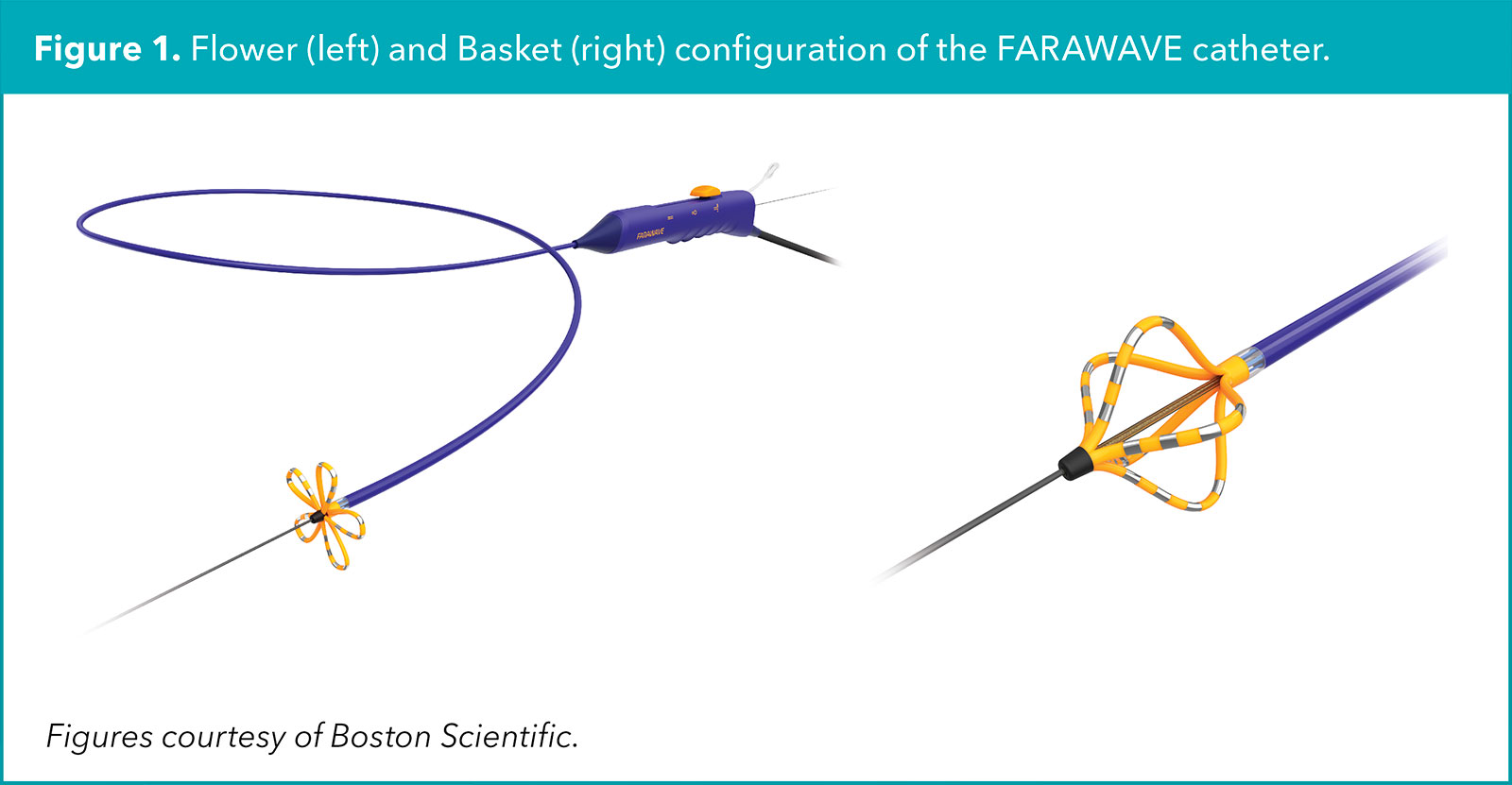
For AFib ablation, adoption of PFA has anecdotally led to less general anesthesia use, shorter procedure duration and greater likelihood of same-day discharge.
While it is unclear whether commercial adoption of PFA in the ventricles will ultimately lead to the same benefits as in the atrium, ongoing animal studies and human case reports for ablation of premature ventricular contractions (PVCs) and ventricular tachycardia (VTs) are promising.
One notable case report described first-in-human histologic lesion analysis in a 60-year-old male with ischemic cardiomyopathy who underwent concomitant PFA and radiofrequency ablation (RFA) for incessant VT, followed by heart transplant 48 hours after the procedure for an established advanced heart failure condition.
A PFA catheter (FARAWAVE, Boston Scientific) was used on the left ventricular (LV) septal wall and an RFA catheter (Qdot Micro, Biosense Webster) was used on the LV anterior wall, which was inaccessible by the PFA catheter. Subsequent histologic analysis showed similar mean lesion depths of 2 mm for both PFA and RFA applications.
However, the RFA lesions exhibited coagulation necrosis with inflammation, edema and hemorrhage, whereas the PFA lesions revealed a homogenous injury pattern similar to cellular apoptosis with preserved cellular membrane. The authors remarked that PFA lesion depth could have been increased with better electrode-tissue contact and lesion stacking.2
More recent human case reports of PFA for the treatment of PVCs and VT have shed new light on PFA use in ventricular arrhythmias.
PFA For PVCs
The first use of PFA in the ventricles was published by Schmidt, et al., in September 2021. The patient was a 48-year-old female with symptomatic PVCs originating from the right ventricular outflow tract (RVOT). Using a basket-configuration catheter (FARAWAVE), PVCs were abolished with the first endocardial PFA application and no PVCs were detected on 48-hour Holter monitoring at the two-month follow-up.3
Subsequent reports of successful PVC ablation with PFA have been described in the endocardium, epicardium and coronary sinus with a focal, contact-force sensing ablation catheter (Centauri, CardioFocus), which is approved for commercial use in Europe but not in the U.S.4-6
The largest case series of focal PFA involved 20 patients with PVCs originating from the RVOT (n=8), LVOT (n=4), moderator band (n=2), LV papillary muscle (n=2), and the RV papillary muscle, tricuspid annulus, aortomitral continuity and left posterior fascicle (n=1 for each). Of the 20 patients, 17 (85%) had no PVC recurrence during 24-hour in-hospital continuous monitoring, two (10%) had significant PVC burden reduction (>80%) during in-hospital monitoring, and one (5%) had no change in PVC burden despite numerous PFA and RFA applications (LVOT PVC origin).
At a median follow-up duration of 120 days, 17 patients (85%) experienced significant PVC burden reduction, including 12 patients (60%) with complete PVC elimination. Periprocedural adverse events included two patients with development of right bundle branch block (moderator band PVC origin), one patient with transient ST segment depression (aortomitral continuity PVC origin), and one patient with femoral artery pseudoaneurysm.7
PFA For VT
In October 2022, Ouss, et al., published the first use of PFA to treat VT, in a 69-year-old male with ischemic cardiomyopathy, prior ICD implant and recurrent symptomatic VT despite prior VT ablation attempts. At the time of the procedure, VT was noninducible but isochronal late activation mapping identified slow conduction zones in the LV distal anteroseptum.
Endocardial PFA was performed via transseptal access using a flower-configuration catheter (FARAWAVE). Remapping after ablation showed absence of prior slow conduction zones. At the six-month follow-up, the ICD showed an absence of recurrent VT.8
Subsequent reports of successful VT endocardial ablation with focal (Centauri) and large footprint (FARAWAVE) PFA catheters have been described via retrograde access, in nonischemic cardiomyopathy patients, in congenital heart disease patients, and to target epicardial substrates.9-12
The only case series of PFA ablation for VT includes three patients at two separate centers using the large footprint (FARAWAVE) PFA catheter. In two patients, PFA at a focal site of the LV midapical lateral wall and at a large aneurysmal site of the LV inferolateral apex resulted in absence of VT recurrence at the six- and five-month follow-up, respectively.
In the third patient with arrhythmogenic cardiomyopathy and a low-voltage substrate in the RV basal inferolateral free wall, numerous PFA applications resulted in dense scar formation, but VT was still inducible (Figure 2).
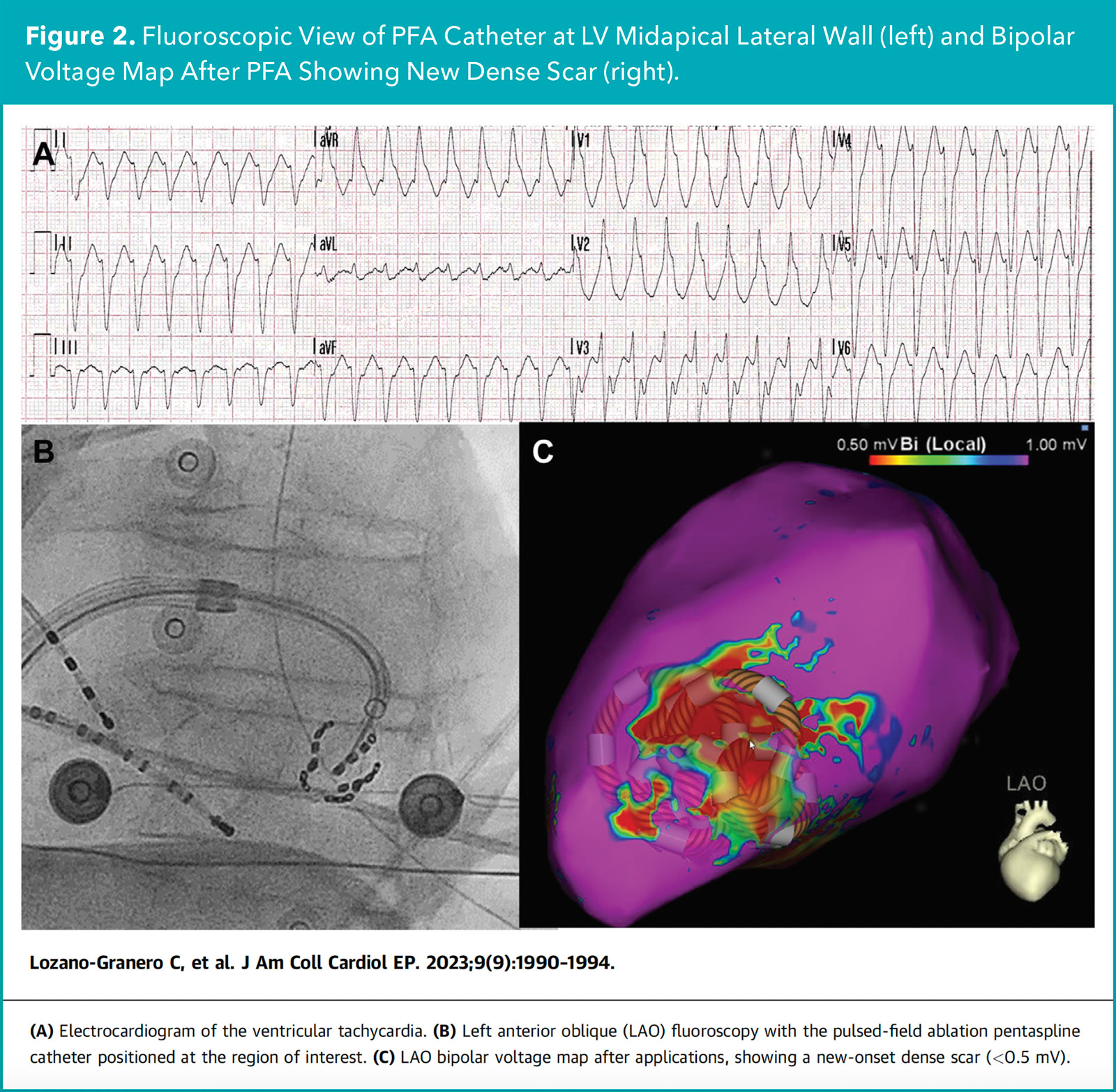
Ultimately, epicardial access and RFA applications in the adjacent epicardial site were needed to achieve noninducibility of further VT. The patient was free of VT recurrence at the four-month follow-up. No periprocedural complications were observed in all three cases.1
The Big Picture
In the relatively short time since CE and FDA approval, PFA has gained widespread adoption over traditional thermal modalities in AFib ablation. I suspect the same trend will occur for ablation of ventricular arrhythmias, particularly as focal PFA catheters become commercially available and PFA waveforms become optimized to target ventricular myocardium.
At the recent 2024 Annual International Symposium on Ventricular Arrhythmias (VT Symposium), an entire session was dedicated to PFA for VT, with live cases showcasing real-world use of emerging PFA technologies in this setting. One day, EPs may approach ablation of PVCs and VTs using PFA with the same ease that we have recently experienced from using PFA for AFib.
Editors' Note: This article discusses evolving technologies and how they may be employed. The use of pulse field ablation in ventricular arrhythmias currently is not approved by the U.S. Food and Drug Administration and it is an off-label indication.

This article was authored by Edward Chu, MD, FACC (@Ed_Chu_MD), an electrophysiology attending physician in Miami, FL.
References
- Lozano-Granero C, Hirokami J, Franco E, et al. Case series of ventricular tachycardia ablation with pulsed-field ablation: pushing technology further (into the ventricle). JACC Clin Electrophysiol 2023;9:1990-4.
- Adragão P, Matos D, Carmo P, et al. Pulsed-field ablation vs radiofrequency ablation for ventricular tachycardia: First in-human case of histologic lesion analysis. Heart Rhythm 2023;20:1395-8.
- Schmidt B, Chen S, Bordignon S, et al. Single shot electroporation of premature ventricular contractions from the right ventricular outflow tract. EP Europace 2022;24:597.
- Worck R, Haugdal MA, Johannessen A, et al. A case of safe and durable focal pulsed-field electroporation treatment of outflow tract premature ventricular contractions. Heart Rhythm O2 2023;4:463-5.
- Mestrovic IP, Breskovic T, Markovic M, et al. Ablation of epicardial ventricular focus through coronary sinus using pulsed‐field ablation. A case report. J Cardiovasc Electrophysiol 2024;35:856-61.
- Spenkelink D, van Wessel H, van Driel VJ, et al. Pulsed field ablation as a feasible option for the treatment of epicardial left ventricular summit premature complex foci near the coronary arteries: a case report. Eur Heart J-Case Reports 2024;8:ytae478.
- Della Rocca DG, Cespón-Fernández M, Keelani A, et al. Focal pulsed field ablation for premature ventricular contractions: A multicenter experience. Circ Arrhythm Electrophysiol 2024;17:e012826.
- Ouss A, van Stratum L, van der Voort P, Dekker L. First in human pulsed field ablation to treat scar-related ventricular tachycardia in ischemic heart disease: a case report. J Interv Card Electrophysiol 2023;66:509-10.
- Martin CA, Zaw MT, Jackson N, et al. First worldwide use of pulsed‐field ablation for ventricular tachycardia ablation via a retrograde approach. J Cardiovasc Electrophysiol 2023;34:1772-5.
- Katrapati P, Weiss JP, Baning D, et al. Pulsed field ablation for incessant scar-related ventricular tachycardia: First US report. Heart Rhythm 2024;21:1236-9.
- Aguilera J, Obeng-Gyimah E, Kuramochi Y, et al. Elimination of epicardial scar-related ventricular tachycardia with endocardial pulsed field ablation: First clinical report. Circ Arrhythm Electrophysiol 2024:e012992.
- Krause U, Bergau L, Zabel M, et al. Flowerpower: pulsed field ablation of ventricular tachycardia in a patient with Ebstein's anomaly. Eur Heart J-Case Reports 2023;7:ytad093.
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Cardiology Magazine, ACC Publications, Ventricular Premature Complexes, Tachycardia, Ventricular, Electrophysiology, Cardiac Electrophysiology
