Innovation in Defibrillator Technology: The Novel OmniaSecure™ Lead
Quick Takes
- The OmniaSecure™ Defibrillator Lead (Medtronic) is a novel, low-caliber, lumenless defibrillator lead that is placed using guide sheaths.
- The OmniaSecure defibrillator lead has high defibrillation success at implantation.
- During 6-12 months of follow-up, the OmniaSecure has high efficacy and a low rate of lead-related complications.
Transvenous leads are the weak link in any implantable transvenous pacemaker or defibrillator system.1 Transvenous leads are associated with vascular occlusions, tricuspid regurgitation, endocarditis, and lead failure. Extraction tools may be needed to remove the leads in the event of a lead-related complication, which can add risk.
Historically, transvenous pacemaker and defibrillator leads have been designed with a central lumen, and stylets are used to place the leads. This central lumen is arguably the source of many of these issues. The hollow lumen may increase the risk of crush injury and lead fracture. The lumen limits how small the diameter of the lead can be. Smaller-caliber leads may be less likely to cause occlusions and tricuspid regurgitation.
Commercially available transvenous defibrillator leads are larger than pacemaker leads. Historically, defibrillator leads were placed in the area of the right ventricular apex, as this was thought to constitute the best defibrillation vector. This location is associated with higher rates of lead perforation compared with a septal position. If the patient requires pacing, this is a nonphysiologic location, associated with risk of pacing-mediated cardiomyopathy.
The SelectSecure™ pacemaker lead (Medtronic) was developed to address some of the issues found with stylet-driven leads.2 This is a 4.1 French (F) solid pacing lead. As there is no central lumen, it is placed using guide sheaths. It has been available for several decades and shows good performance.3,4 It is commonly used for conduction system pacing.5
The OmniaSecure™ Defibrillator Lead (Medtronic) was built on the platform of the SelectSecure lead (Figure 1). This lead is 4.8F in diameter and has a single defibrillation coil with integrated sensing. The LEADR (Lead EvaluAtion for Defibrillation and Reliability) trial was the pivotal trial evaluating the OmniaSecure lead. This global study of 675 patients was conducted in 45 sites over a 12-month period. The primary efficacy endpoint was defibrillation success at implantation. The primary safety endpoint was freedom from lead-related complications at 6 months.
Figure 1: Schematic Comparison Between the OminaSecure™ Defibrillator Lead, the SelectSecure™ Pacing Lead, and the Sprint Quattro™ Defibrillator Lead
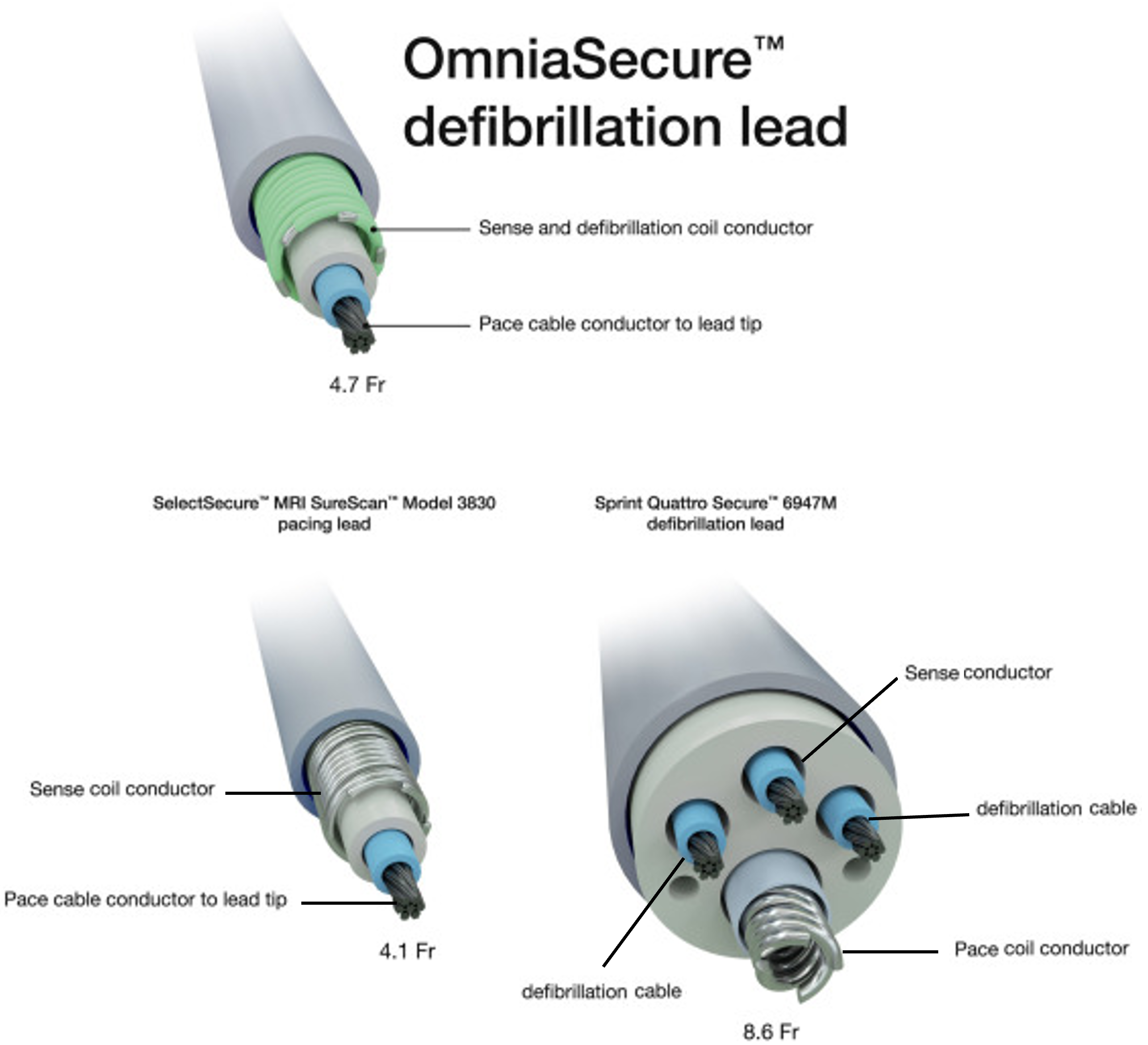
Reprinted with permission from Medtronic (CC BY 4.0).
The results of the LEADR trial were presented as a late-breaking trial at the 2024 Heart Rhythm Society (HRS) scientific sessions and simultaneously published in the journal Heart Rhythm.6 Implantation success rate was 97.9%. Defibrillation efficacy was 97.5%, exceeding the prespecified endpoint. Freedom from lead-related complications was 97.1% at 6 months. Electrical parameters remained stable, and no fractures occurred. Implanting clinicians were able to place 99.5% of successfully implanted leads in the desired location. There was a secondary safety endpoint that used a previously published simulation model in combination with the clinical data to provide a 12-month fracture-free estimate, which was 99.97%. In addition, 95% of appropriate shocks were successful during the follow-up period. The inappropriate shock rate was <3%, with atrial arrhythmias as the most common cause. T-wave oversensing caused three inappropriate shocks, and none were caused by p-wave oversensing.
The computer simulation aspect of this study is worth consideration. Defibrillator trials are expensive to perform, and many lead complications occur late. A validated reliability model was used and new biplane fluoroscopy data from the trial included. The investigators have subsequently published the projected 10-year lead reliability modeling at 98.2%.7 This modeling may become an increasingly common manner to evaluate novel pacing and defibrillator leads.
There are possible benefits associated with the OmniaSecure lead compared with stylet-driven defibrillator leads. It could be speculated that this smaller-caliber lead is less likely to cause venous thrombosis and stenosis, tricuspid regurgitation, and infection. It might have favorable characteristics for extraction.8 The most intriguing use of this lead is for placement in the conduction system to provide both defibrillation and a form of cardiac resynchronization therapy. The LEADR LBBAP (Lead Evaluation for Defibrillation and Reliability in LBBAP) trial is currently underway to address whether the OmniaSecure lead provides successful defibrillation in this location. More investigation will be needed to determine the full benefits of this product.
References
- Maisel WH. Transvenous implantable cardioverter-defibrillator leads: the weakest link. Circulation. 2007;115(19):2461-2463. doi:10.1161/CIRCULATIONAHA.107.698597
- Gammage MD, Lieberman RA, Yee R, et al. Multi-center clinical experience with a lumenless, catheter-delivered, bipolar, permanent pacemaker lead: implant safety and electrical performance. Pacing Clin Electrophysiol. 2006;29(8):858-865. doi:10.1111/j.1540-8159.2006.00452.x
- Chakrabarti S, Morgan GJ, Kenny D, et al. Initial experience of pacing with a lumenless lead system in patients with congenital heart disease. Pacing Clin Electrophysiol. 2009;32(11):1428-1433. doi:10.1111/j.1540-8159.2009.02487.x
- Kenny D, Walsh KP. Noncatheter-based delivery of a single-chamber lumenless pacing lead in small children. Pacing Clin Electrophysiol. 2007;30(7):834-838. doi:10.1111/j.1540-8159.2007.00769.x
- Vijayaraman P, West M, Dresing T, et al. Safety and performance of conduction system pacing: real-world experience from a product surveillance registry. Heart Rhythm. 2025;22(2):318-324. doi:10.1016/j.hrthm.2024.06.061
- Crossley GH 3rd, Sanders P, Hansky B, et al. Safety, efficacy, and reliability evaluation of a novel small-diameter defibrillation lead: global LEADR pivotal trial results. Heart Rhythm. 2024;21(10):1914-1922. doi:10.1016/j.hrthm.2024.04.067
- Crossley GH 3rd, Mason PK, Hansky B, et al. High predicted durability for the novel small-diameter OmniaSecure defibrillation lead. Heart Rhythm. 2025;22(2):302-310. doi:10.1016/j.hrthm.2024.09.005
- Vatterott PJ, Mondésert B, Marshall M, Lulic T, Wilkoff BL. Mechanics of lumenless pacing lead strength during extraction procedures based on laboratory bench testing. Heart Rhythm. 2023;20(6):902-909. doi:10.1016/j.hrthm.2023.02.025
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias
Keywords: Electric Countershock, Defibrillators, Implantable