Not All ICD Complications Are Created Equal: Insights From the PRAETORIAN-XL Trial
Quick Takes
- The PRAETORIAN-XL (A Prospective, Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter Defibrillator Therapy—Long-Term Follow-Up) trial results demonstrated no differences in all complications between transvenous implantable cardioverter-defibrillators (ICDs) and subcutaneous ICDs during an additional 4 years of follow-up after the original trial.
- Major complications, including lead-related complications, were significantly higher in the transvenous ICD group.
Defibrillator leads are the weak link in traditional transvenous implantable cardioverter-defibrillator (TV-ICD) systems. Cardiac perforation, pneumothorax, thrombosis, infections, and lead malfunction are some of the short- and long-term complications associated with transvenous lead use.1 New innovations in device therapy have made strides to avoid these issues. The subcutaneous implantable cardioverter-defibrillator (S-ICD) has been available for over a decade and was one of the first devices brought to market that did not use traditional transvenous leads.
S-ICD devices are completely extracardiac and extravascular. The design consists of the generator, which is implanted in the axillary area; a lumenless implantable cardioverter-defibrillator (ICD) lead, which is tunneled subcutaneously between the generator; and an incision near the xiphoid process, where it is anchored. The tip of the lead is tunneled subcutaneously toward the suprasternal notch (Image 1). S-ICDs do not provide either bradycardia pacing therapy or antitachycardia pacing to treat ventricular tachycardia.2
Image 1: Lateral CXR Demonstrating the Position of the S-ICD Generator and Lead
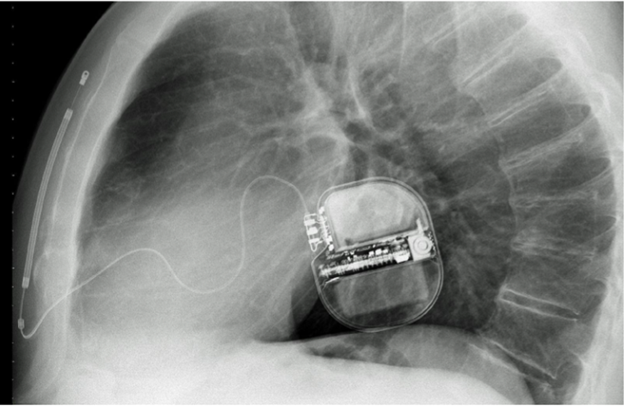
CXR = chest radiograph; S-ICD = subcutaneous implantable cardioverter-defibrillator.
The PRAETORIAN (A Prospective, Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter Defibrillator Therapy) trial results demonstrated noninferiority of S-ICDs compared with traditional TV-ICDs over a median follow-up of 49.1 months.3 Performed as an extension, the PRAETORIAN-XL (A Prospective, Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter Defibrillator Therapy—Long-Term Follow-Up) trial compared the long-term follow-up complications between TV-ICDs and S-ICDs. The trial took place across 39 centers in the United States and Europe over an additional 4 years from December 2019 to December 2023. Of the original 849 patients, 528 were included in the PRAETORIAN-XL trial. The primary endpoint was any device-related major or minor complication. Major complications were defined as those requiring an invasive procedure.
Results of the PRAETORIAN-XL trial showed no statistically significant differences in overall complications in patients with TV-ICDs versus those with S-ICDs (11.6% vs. 8%; p = 0.15) in the modified intention-to-treat analysis.4 There was, however, a higher risk of major and lead-related complications in patients with TV-ICDs than in those with S-ICDs (10.2% vs. 5.7%; p = 0.03). This finding corresponds to earlier data from the ATLAS (Avoid Transvenous Leads in Appropriate Subjects) trial.5 The differences were even greater between patients with TV-ICDs and those with S-ICDs when evaluating lead-related complications alone (8.3% vs. 2.4%; p < 0.001). The crossover rate was twice as high in patients with S-ICDs as in those with TV-ICDs, likely because patients developed pacing needs.
These findings are particularly striking because there were fewer complications than anticipated in the TV-ICD group. This finding may have been due to lower rates of generator change, which is when many complications occur, in patients with TV-ICDs in the PRAETORIAN-XL trial. Longer-term follow-up may expose more lead-related complications. In addition, there was a higher frequency of generator changes with S-ICDs in this trial due to recall-related battery depletion.
The PRATORIAN-XL investigators concluded that S-ICDs should be considered in patients requiring ICD therapy without a known indication for pacing given the low rates of major complications. The recently released extravascular ICD presents another option for ICD therapy while avoiding transvenous leads.6 Clinical understanding will continue to deepen as experience with these devices grows. However, the PRAETORIAN-XL trial results contribute to the body of data that shows that avoiding transvenous leads, when possible, improves patient outcomes.
References
- Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553-1561. doi:10.1161/CIRCULATIONAHA.110.976076
- Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36-44. doi:10.1056/NEJMoa0909545
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383(6):526-536. doi:10.1056/NEJMoa1915932
- Olde Nordkamp LRA, de Veld JA, Ghani A, et al. Device-related complications in transvenous versus subcutaneous defibrillator therapy during long-term follow-up: the PRAETORIAN-XL trial. Circulation. 2025;152(3):172-182. doi:10.1161/CIRCULATIONAHA.125.074576
- Rordorf R. The ATLAS randomised clinical trial: what do the superiority results mean for subcutaneous ICD therapy and sudden cardiac death prevention as a whole?. Arrhythm Electrophysiol Rev. 2022;11:Suppl 1. doi:10.15420/aer.2022.11.S1
- Friedman P, Murgatroyd F, Boersma LVA, et al. Efficacy and safety of an extravascular implantable cardioverter-defibrillator. N Engl J Med. 2022;387(14):1292-1302. doi:10.1056/NEJMoa2206485
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Electrophysiology, Cardiac Electrophysiology, Defibrillators, Implantable