PULSAR: Novel PFA Device Safe, Effective For PVI in Patients With PAF
A spherical multielectrode pulsed field ablation (PFA) catheter enabled safe, efficient and durable pulmonary vein (PV) isolation (PVI) in paroxysmal atrial fibrillation (PAF), according to results from the PULSAR trial published Jan. 21 in JACC.
In the prospective, multicenter, single-arm pivotal investigational device exemption trial, 183 patients (average age, 65 years; 40% women, average CHA2DS2‑VASc score, 2.1; average left atrial diameter, 3.8 cm) with symptomatic drug-resistant or intolerant PAF underwent PVI with the use of the spherical array system.
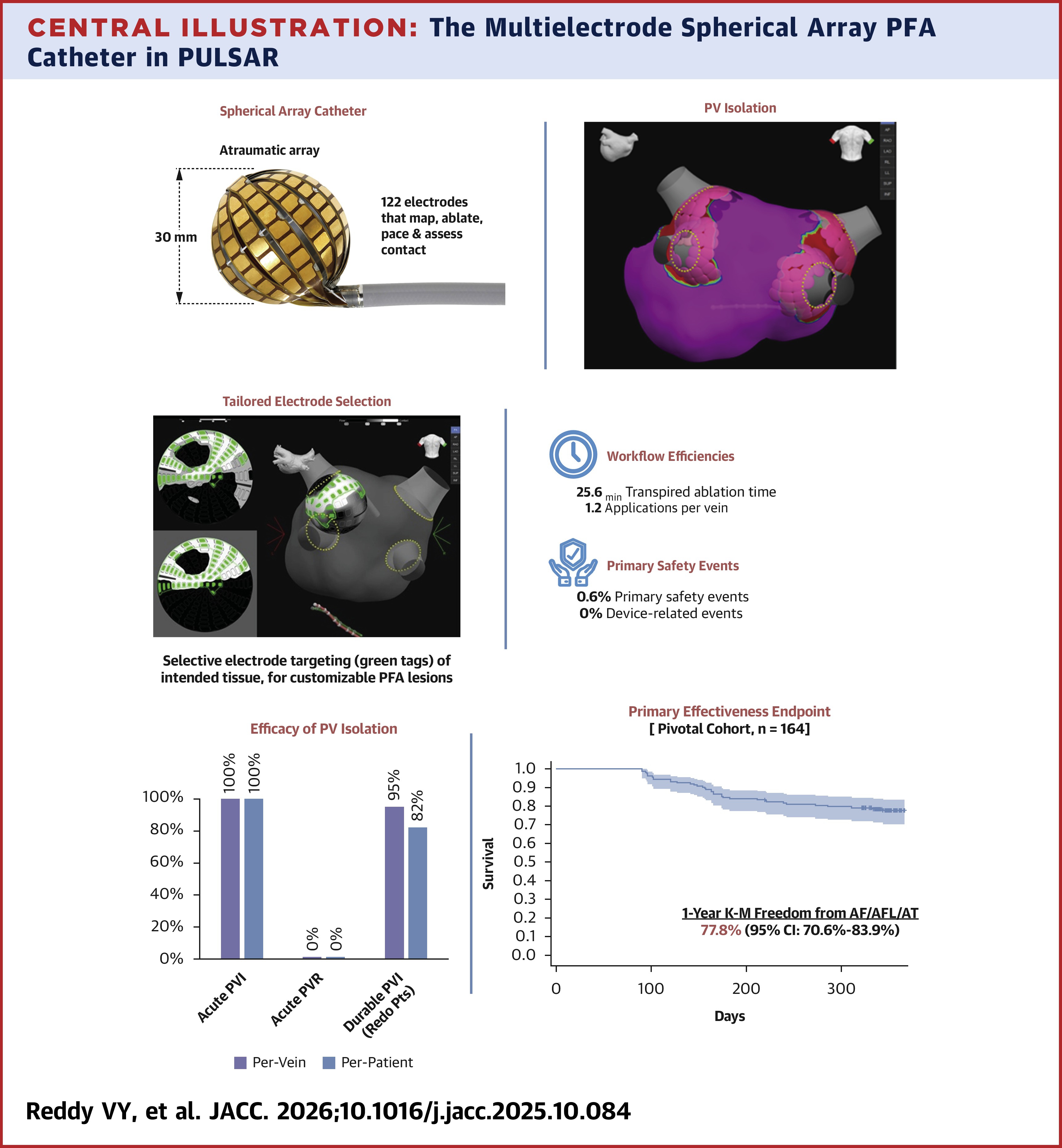
Results showed that all 647 veins were successfully and accurately isolated, with 94% isolated with a single 40-second PFA application. Also, the procedure was efficient, with a mean mapping time of 9.0 minutes, transpired ablation time of 25.6 minutes, left atrial dwell time of 59.9 minutes and overall procedure time of 95.8 minutes. Mean fluoroscopy time was 9.2 minutes.
Furthermore, the procedure was safe. At 12 months, 78% of patients were free from treatment failure and 84% were free from symptomatic arrhythmias. Only 6% required re-ablation. During redo mapping, 95% of veins remained isolated and 82% of patients showed complete, durable PVI.
Only one patient suffered a primary safety event – a single hemorrhagic stroke in a patient with severe hypertension and polycystic kidney disease. There were no device-related events, or any instances of PV stenosis, atrio-esophageal fistula, permanent phrenic nerve paralysis, cardiac tamponade or embolic stroke. A PV stenosis substudy of 33 patients found no PV narrowing at six months.
Study authors Vivek Y. Reddy, MD, et al., note that the device comes with a favorable learning curve: "once the multielectrode spherical array is pushed up against a PV, the electrodes to deliver the PF energy are selected electronically, that is, without requiring any of the technical skill typically required for catheter manipulation."
In an accompanying editorial comment, Boris Schmidt, MD, and K.R. Julian Chun, MD, called the outcome "remarkable."
"The most innovative potential of the global array lies in the capability to simultaneously record intracardiac electrograms from 122 distinct electrodes. This will allow us to improve our understanding of electrical phenomena present during atrial fibrillation and may help to identify drivers, rotors, and other arrhythmogenic sources," they write. "Thus, it may be a unique mapping and ablation solution for patients with persistent [atrial fibrillation] or with [atrial fibrillation] independently from the pulmonary veins."
Clinical Topics: Arrhythmias and Clinical EP, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Pulmonary Veins, Catheter Ablation, Atrial Fibrillation, Electrophysiology
< Back to Listings
