AZALEA-TIMI 71: Should Abelacimab Be Withdrawn Before Invasive Procedures?
In patients with atrial fibrillation (AFib) randomized to abelacimab vs. rivaroxaban, no statistically significant differences in bleeding risk were observed, suggesting that interruption of abelacimab treatment may not be necessary before invasive procedures with low bleeding risk, according to a perioperative subanalysis of the AZALEA-TIMI 71 trial published in JACC.
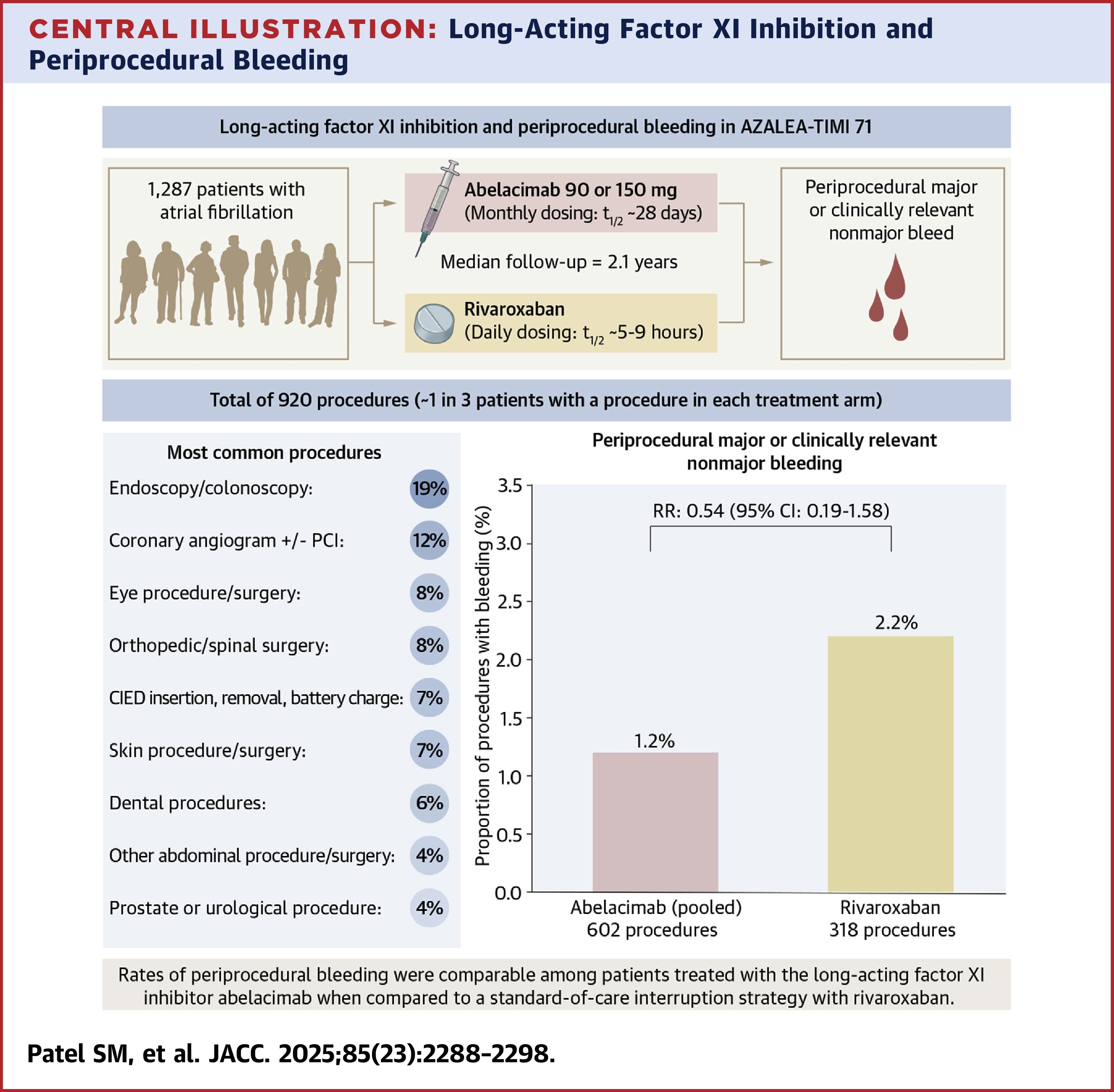
In this phase 2b, dose-ranging study, patients with AFib (median age 74 years and 20% >80 years; 45% women; 95% White) were randomized to either abelacimab, a novel factor XI inhibitor (at 90 or 150 mg subcutaneous doses monthly) or daily rivaroxaban. The trial was double blind with regard to the abelacimab doses but open label for abelacimab vs. rivaroxaban.
A primary focus of the subanalysis was periprocedural bleeding events identified as major or clinically relevant nonmajor (CRNM) bleeds, as adjudicated by a clinical events committee blinded to treatment assignment, occurring within 30 days of procedure and related to the procedure as determined by blind review.
Results showed that, during a median follow-up of 2.1 years, 34% of patients in the abelacimab arms underwent a total of 602 procedures while 36% of patients in the rivaroxaban arm underwent a total of 318 procedures.
The occurrence of periprocedural major or CRNM bleeding was low, accounting for <2% of all procedures, including 1.2% of all procedures in the abelacimab arm and 2.2% of all procedures in the rivaroxaban arm (risk ratio 0.54). Additionally, major or CRNM bleeds occurred in only three of 366 procedures (0.9%) occurring within 30 days of abelacimab dose. No fatal bleeding events occurred in either arm.
Of the 920 total procedures, 76% were low bleeding risk and 75% were elective. The most common procedures were endoscopy or colonoscopy (19%), coronary angiogram with or without PCI (12%) and ocular procedures or surgeries (9%). The most common high-risk procedures were major orthopedic surgery (32%), spinal surgery (31%) and abdominal surgery (12%). Hemostatic therapies were used in 39 (7%) of procedures in the abelacimab arms compared with eight procedures (3%) in the rivaroxaban arm, with the most common being an antifibrinolytic agent like tranexamic acid.
Siddarth M. Patel, MD, MPH, et al., note that one in five patients on anticoagulation therapy will need an elective surgery or invasive procedure, currently necessitating sophisticated direct oral anticoagulant management and withdrawal – with abelacimab's long 28-day half-life complicating matters considerably. "These findings suggest that routine interruption of anticoagulation may not be necessary for all procedures with factor XI inhibition, particularly procedures that are low bleeding risk," they write.
In an accompanying editorial comment, James D. Douketis, MD; P. Gabriel Steg, MD, FACC; Suzanne C. Cannegieter, MD, PhD; and Alex C. Spropoulos, MD, note there are still many unanswered questions regarding procedures performed during factor XI inhibition, but that the study is "a small but important step toward changing the paradigm about what constitutes adequate perioperative hemostasis," emphasizing that several such steps are needed to "direct us toward a giant leap of clinical acceptance and practice change."
An editor's note by Behnood Bikdeli, MD, FACC, adds that, "Enthusiasm persists about factor XI/XIa inhibition for the continued hope of uncoupling thrombosis from hemostasis. The key element for that premise is proof of effectiveness." He notes that, "Findings from the ongoing trials will be instrumental in shaping our understanding. Until then, the study by Patel et al., has moved us one step further about the promising safety of these agents."
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Anticoagulation Management and Atrial Fibrillation, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Factor XI, Fibrinolytic Agents, Atrial Fibrillation, Perioperative Care, Anticoagulants
< Back to Listings
