Cover Story | Navigating AFib Care Across the Continuum: Insights From the Guideline

The ACC and the American Heart Association (AHA) guideline for the management of atrial fibrillation (AFib) provides a comprehensive multi-society update, the first in a decade, and offers a new staging system for AFib, upgrades recommendations for catheter ablation, rhythm control and left atrial appendage occlusion (LAAO), and emphasizes the importance of managing risk factors both in the prevention and treatment of the arrythmia, and more.1
The 2023 guideline introduces innovative approaches to AFib classification, anticoagulation therapy and rhythm control, reflecting a more nuanced understanding of AFib's pathophysiology than previous guidance. Notably, it highlights the importance of lifestyle modifications and the integration of digital health tools in managing this complex arrhythmia.
With AFib affecting an estimated 50 million people in 2020 and its prevalence and incidence increasing in the U.S. and globally, this guideline is a crucial tool for clinicians striving to improve patient outcomes.
AFib as a Continuum
AFib is the most common arrhythmia and accounts for significant morbidity and mortality. Whereas AFib was previously classified based only on arrhythmia duration (paroxysmal, persistent, longstanding persistent) and only once diagnosed, the new guideline recognizes AFib as a disease continuum that starts with patients at-risk but without diagnosed AFib (Stage 1) and progresses to those in a permanent state of AFib (Stage 4).
At each stage, a variety of strategies are proposed ranging from heightened surveillance and risk factor modification, to stopping attempts at rhythm control after a shared decision-making discussion between patient and clinician for those in Stage 4 or permanent AFib.
"The different stages better define AFib as a progressive disease and highlight the need to address it at the earliest stages, especially emphasizing the importance of prevention, risk factor management, and timing for screening in those patients at the highest risk," write the guideline authors.
The staging system will be particularly beneficial for individuals in Stage 2 (pre-AFib) who have evidence of structural or electrical abnormalities that predispose them to AFib, says Hugh Calkins, MD, FACC, director of electrophysiology at Johns Hopkins Medicine in Baltimore.
"We often see people who are getting little bursts of atrial tachycardia, not enough to call it AFib, but their chances of getting AFib are high if followed over time," he says. The new staging scheme improves the recognition and management of patients with bursts of atrial tachycardia, an important objective, and highlights the need to follow them and "get serious about risk factor modification so we can possibly prevent the AFib."
"I call AFib the low back pain of cardiology – it's common, chronic, hard to manage, and we don't have great therapies for it," says Fred M. Kusumoto, MD, FACC, who served on the guideline writing committee and is chair of the Heart Rhythm Division at Mayo Clinic in Jacksonville, FL.
With the staging system, he adds, the guideline authors hope to interrupt the relentlessly growing impact of AFib, recognizing that in many people the disease can be prevented or slowed through risk factor modification.
"The public health burden of AFib is increasing," says Kusumoto. "Therefore, it is important to be thoughtful in our approach to modifying risk factors for prevention, considering that this approach can be applicable to a large proportion of the population. There is strong evidence that AFib is largely a lifestyle disease that we may be able to prevent, so it is appropriate to be ambitious in our prevention efforts."
Modifiable Risk Factors Front and Center
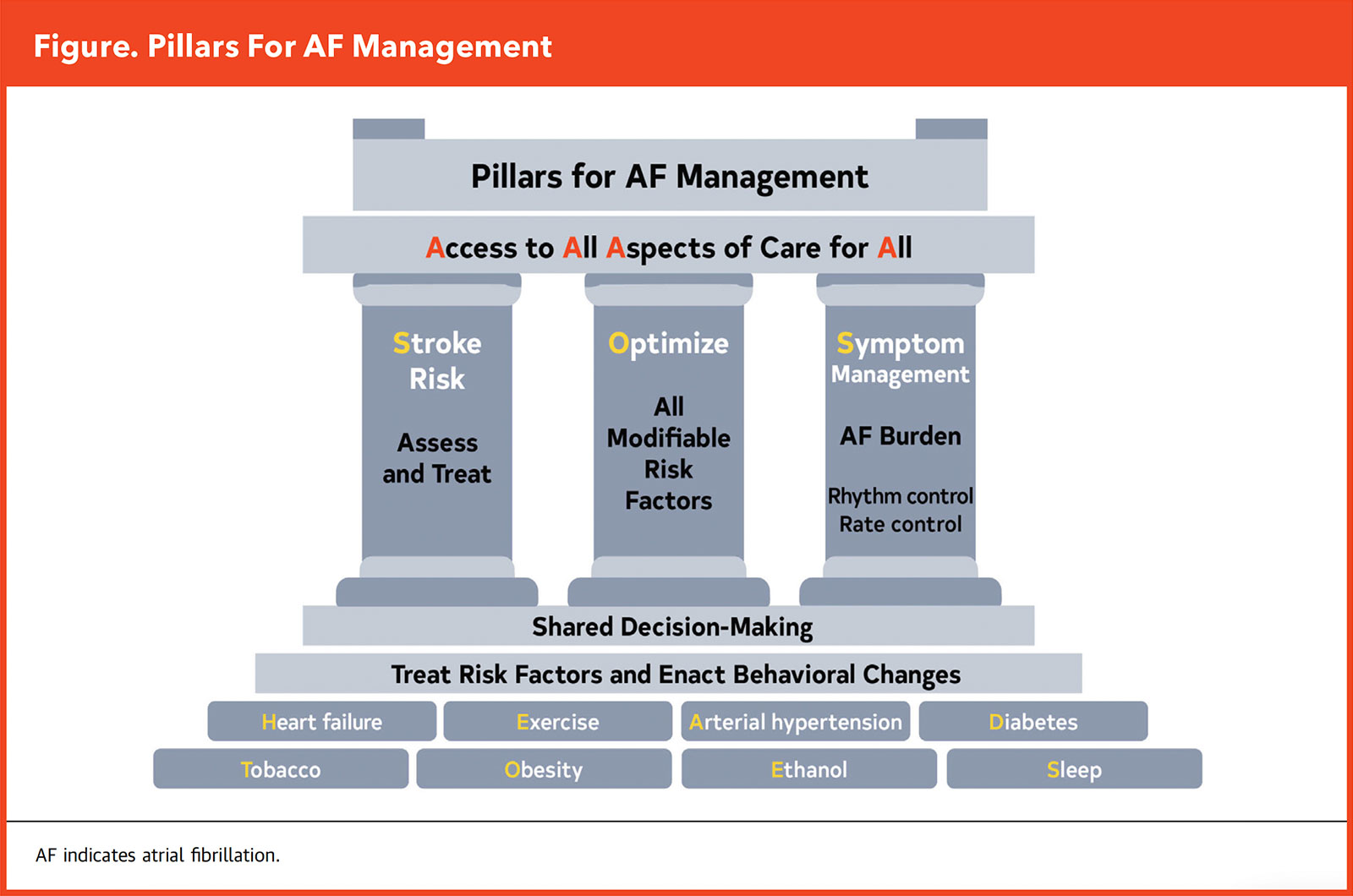
"Lifestyle and risk factor modification is front and center in the new guideline – serving as the central pillar of our three pillars of AFib management," says Prashanthan Sanders, MBBS, PhD, a writing committee member, from the Centre for Heart Rhythm Disorders, University of Adelaide in Australia (Figure). "The global prevalence of AFib is increasing largely as a result of elevated levels of modifiable risk factors," he adds.
Reinforcing the significance of implementing early lifestyle interventions and specific modifications to prevent the onset and progression of AFib, the foundation of the three pillars is a holistic, comprehensive risk screening of every patient, guided by the HEAD 2 TOES acronym provided in the guideline: heart failure, exercise, arterial hypertension, diabetes type 2, tobacco smoking, obesity, ethanol consumption, and sleep apnea.
– Hugh Calkins, MD, FACC
The guideline also provides targets for these risk factors for secondary prevention, including 210 minutes a week of moderate-to-vigorous exercise, smoking cessation with guideline-directed medical treatment (GDMT) as needed, weight loss of at least 10% for those with overweight or obesity, minimizing or eliminating alcohol consumption, and screening for sleep apnea, along with optimal control of blood pressure and diabetes.
Pressed as to which risk factor is most important, Sanders suggests it's a mistake to single out any of them. "The most important risk factor is the one that is most predominant and relevant to the specific patient," he says.
The development of AFib is complex, usually involving years of exposure to multiple risk factors, leading to the development of an advanced substrate that predisposes to AFib initiation, persistence and progression. "If you attend to this relatively limited list of risk factors, you will make a difference in your patient's clinical course."
Moreover, "we have stressed in the guideline that risk factor management is relevant across all four stages of AFib. From the research we have, the biggest bang for the buck is upstream, in patients at risk for AFib," says Kusumoto.
More Nuanced Use of Anticoagulation
In the ACC/AHA AFib guideline, recommendations for anticoagulation are made based on annual thromboembolic event risk using validated risk scores, including CHA2DS2-VASc as one option, but with flexibility in using other clinical risk scores (e.g., ATRIA or GARFIELD-AF) and considering other risk variables to help inform shared-decision making.
For patients with AFib and an annual risk of ischemic stroke of 2%, oral anticoagulation is strongly recommended. For patients at intermediate to low (<2%) annual risk of ischemic stroke, clinicians should consider "stroke modifiers" to inform shared decision-making.
These additional stroke risk factors, none of which are included in the CHA2DS2-VASc score, include higher AFib burden and longer duration, persistent or permanent AFib (vs. paroxysmal), obesity, hypertrophic cardiomyopathy, reduced kidney function, poorly controlled hypertension or enlarged left atrial size.
– Fred M. Kusumoto, MD, FACC
"This recommendation is consistent with my clinical practice," says Calkins. "If I see a man with CHA2DS2-VASc 1 score or a woman with CHA2DS2-VASc 2 score, I don't stop there. Rather, I think about how large is their left atrium, what's their AFib burden, is their blood pressure controlled?" He notes this guideline recommendation to consider additional stoke risk factors reflects "the recognition that the CHA2DS2-VASc is very imperfect and decisions regarding anticoagulation are not so simple."
With this, a key theme in the 2023 guideline is the emphasis on shared decision-making and patient-centered care. Clinicians are encouraged to discuss the benefits and risks of anticoagulation with patients, considering their preferences, lifestyle and specific clinical circumstances.
Of note, the guideline favors direct oral anticoagulants (DOACs) over vitamin K antagonists for most patients due to their ease of use, predictable pharmacokinetics and lower risk of major bleeding. Warfarin remains an option for patients with mechanical heart valves, moderate-to-severe mitral stenosis or those who have contraindications to DOACs.
Ablation Expanded
"Besides the lifestyle part, I think the other real contribution of this new guideline is the emphasis placed on early rhythm control. There are accumulating data suggesting that we should intervene early to prevent the progression of AFib," says Christine M. Albert, MD, MPH, FACC. Albert is chair of the cardiology department at Smidt Heart Institute at Cedars-Sinai in Los Angeles.
Practice Support
ACC's AFib Guideline Hub provides guideline-related resources for clinicians and patients, including the Guideline-at-a-Glance, the JACC Central Illustration and JACC Interactive Tool on Catheter Ablation as First-Line Therapy for Rhythm Control, as well as the Guideline Clinical App and more. Click here to access it all. Be sure to download the free CardioSmart infographics explaining AFib as well as medications, along with an action plan, decision aids and fact sheet.

Consistent with that is a marked expansion of the indications for catheter ablation. These changes are based on recent randomized studies that have demonstrated the superiority of catheter ablation over drug therapy for rhythm control as the treatment modality for maintaining sinus rhythm.
A Class I recommendation for catheter ablation has been given for three groups: 1) patients with symptomatic AFib refractory to antiarrhythmic drug therapy; 2) as first-line therapy for selected patients (generally younger, fewer comorbidities) with symptomatic paroxysmal AFib in whom rhythm control is desired; and 3) patients with heart failure with reduced ejection fraction (HFrEF) on GDMT.
Catheter ablation has received a Class IIa indication as first-line therapy for three groups: 1) patients with symptomatic paroxysmal or persistent AFib; 2) selected patients with asymptomatic or minimally symptomatic AFib in whom catheter ablation may be useful for reducing progression of AFib and its associated complications; and 3) patients with heart failure with preserved ejection fraction (HFpEF).
"The guideline offers clear recognition that catheter ablation now is mainstream treatment of AFib. But it's important to note that we have also said that pulmonary vein isolation is recommended as the primary lesion set for all patients unless another target or a specific trigger is identified," says Kusumoto.
"Results have been mixed for other ablation targets, like the LAA, posterior wall, atrial scar, etc., and no other strategy has emerged that is broadly applicable in all patients," he adds.
Catheter Ablation in Heart Failure
One of the major highlights in the new guideline is the upgraded recommendations for catheter ablation in patients with HF, says Albert. For patients with HFrEF, the guideline gives a Class I recommendation for catheter ablation based on recent studies that have shown the superiority of ablation over drug therapy for rhythm control in these patients.
AFib and HF often coexist, and both can contribute to the development of the other. AFib also worsens outcomes in HF, particularly HFrEF.
"When someone is diagnosed with a new cardiomyopathy and has AFib, we try to get them into sinus rhythm in the hopes that their left ventricular ejection fraction (LVEF) and HF symptoms will improve," says Albert. Research shows that obtaining sinus rhythm, especially through ablation, improves LVEF and improves outcomes in patients with HFrEF, she notes.
– Christine M. Albert, MD, MPH, FACC
"Allowing AFib to persist long term, regardless of reasonable rate control, may result in worsening HF and cardiomyopathy. An early and aggressive approach to rhythm control can reduce AFib burden, resulting in favorable ventricular remodeling and halting of any occult arrhythmia-induced cardiomyopathy," write the guideline authors.
As for HFpEF, the evidence supporting catheter ablation is not as strong and it receives a Class IIa recommendation to improve symptoms and quality of life. In a prespecified subanalysis of the EAST-AFNET trial, most of whom were patients with HFpEF, early rhythm control significantly improved the composite outcome of death, stroke, or hospitalization for worsening of HF or for an acute coronary syndrome, compared with usual care.
LAAO Gets a Modest Boost
Percutaneous LAAO first appeared in the 2019 ACC/AHA guideline update as a Class 2B recommendation in patients with increased risk of stroke who have nonreversible contraindications to long-term oral anticoagulation. Now, the recommendation has been upgraded to Class 2a for this patient population.
"The big issue here is that everyone is really waiting for two big trials – CATALYST and CHAMPION-AF – which are looking at LAAO being noninferior to a DOAC in those able to tolerate the drug option," says Kusumoto. "Results from these studies will be out in a couple of years and then we'll have more to say about whether we can expand the indication for LAAO."
For those with AFib and a moderate-to-high risk of stroke and a high risk of major bleeding on oral anticoagulation, LAAO is a reasonable alternative to oral anticoagulation based on patient preference, with careful consideration of procedural risk and with the understanding that the evidence for oral anticoagulation is more extensive.
In patients undergoing cardiac surgery, surgical LAA exclusion is indicated for those at high risk of stroke (Class I). The guideline authors note, however, that anticoagulation should be continued in these patients even after surgical LAAO. The benefit of surgical LAAO in the absence of continued anticoagulation to reduce the risk of stroke and systemic embolism is certain.
Wearables Have a Role in Care
Click here for the JACC interactive tool to identify the patients most likely to benefit from catheter ablation as first-line therapy.
Click here to read more about using wearables for AFib.
With the new ruling from the U.S. Food and Drug Administration (FDA) in May, the Apple Watch has clearly advanced beyond the realm of fun gadget. The agency ruled that the Watch's AFib history feature, which records and alerts the wearer's relevant heart events, can be used to help evaluate estimates of AFib burden as a secondary effectiveness endpoint within clinical studies intended to evaluate the safety and effectiveness of cardiac ablation devices.
Said the agency, "The weekly estimates of AFib burden generated by the AFib History Feature can be used for comparative analysis across arms of a clinical study. The AFib History Feature may be used throughout the clinical study to monitor a participant's weekly estimate of AFib burden in order to compare weekly burden estimates."
The FDA qualifies, however, that the Watch's history feature cannot by itself be used to evaluate the safety and effectiveness of cardiac ablation devices. In other words, it should not replace the findings of any primary endpoints.
Regarding wearable devices and other continuous monitoring technologies, the AFib guideline authors offer a few insights: First, the consumer-driven use of these devices has blurred the line between clinical and subclinical AFib and is increasing the diagnosis of AFib in younger individuals.
Second, wearables are a reasonable means of monitoring AFib burden and recurrence after interventions against AFib, both in the short and longer terms. Third, the best ways to use wearables in screening patients for AFib are yet to be defined.
The guideline authors also updated the recommendations for device-detected AFib to consider the interaction between episode duration and the patient's underlying risk for thromboembolism. This includes considerations for patients with AFib detected via implantable devices and wearables.
Emphasizing the need for continual updating of the guideline, Albert suggests the recommendations don't quite go far enough in terms of recommending anticoagulation for individuals with subclinical AFib of longer duration.
"The guideline gives a Class IIa recommendation for device-detected high-rate AFib lasting at least 24 hours. But I think there have been a few substudies more recently that give us more certainty in this area and might warrant upgrading that recommendation to Class I."
Comprehensive Guidance
The 2023 ACC/AHA AFib guideline, which spans a record 171 pages, is a comprehensive guide to managing individuals at risk for and with AFib across a wide range of scenarios. The guideline writers, says Sanders, tried where possible to be forward thinking. "For example, the GARFIELD-AF risk score includes about 40 variables, which is far more than a clinician can manage at the bedside. However, we fully expect these risk scores will soon be embedded within the electronic medical record."
"Overall, the authors have hit the mark," says Calkins. "This is a very refreshing, strong document that provides guidance that is consistent with my clinical practice. I am impressed with it."
This article was authored by Debra L. Beck, MSc.
Reference
- Writing Committee Members, Joglar JA, Chung MK, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation. J Am Coll Cardiol 2024;83:109-279.

Pulsed Field Ablation
Pulsed field ablation (PFA) is emerging as an alternative to thermal ablation of AFib, thanks to its precision and safety. PFA employs high-voltage electrical pulses to create microscopic pores in cell membranes, selectively targeting myocardial tissue while sparing adjacent structures from thermal injury, including the esophagus, phrenic nerve and blood vessels.
One of the great hopes for PFA is that its selective and nonthermal targeting reduces the risk of atrioesophageal fistula, a relatively rare event that can result in death.1 Additionally, PFA procedures are generally quicker, thereby reducing overall procedure time and improving patient throughput in clinical settings.
With a head start in Europe, PFA is coming to the US. To date, the FDA has approved two PFA systems: Medtronic's PulseSelect PFA system in December 2023 and Boston Scientific's Farapulse System in January 2024. The PulseSelect system has an indication for both paroxysmal and persistent AFib, while the Farapulse is indicated for the treatment of paroxysmal AFib. Farapulse was approved in the EU in 2021.
In the recent ADVENT trial, 607 patients with drug-refractory paroxysmal AFib were randomized either to PFA with the Farapulse system or to thermal ablation (either radiofrequency or cryoballoon).2
At one year, PFA was shown to be noninferior to thermal ablation in respect to efficacy (a composite of acute procedural and chronic success) and safety (device-related and procedure-related serious adverse events). PFA did not achieve superiority.
After one year of follow-up, 73.3% of the patients who underwent PFA and 71.3% of those who underwent thermal ablation were free of recurrent atrial arrhythmias, a result considered "disappointing" for PFA given the lack of incremental improvement.1 Mean total procedure time was shorter in the PFA group (106 vs. 123 minutes). Mean fluoroscopy time was 21 and 14 minutes, respectively.
A new analysis of the trial, just presented at Heart Rhythm 2024 and published in JACC, suggests that PFA offers a potential improvement over thermal ablation in reducing residual AFib burden, a metric that correlates with better clinical outcomes and quality of life than the traditional 30-second recurrence metric.3
"EPs are excited about PFA because it makes the procedure shorter, and as operators gain more experience, I expect the procedure time differences will become even greater," says Albert.
"There are some data suggesting better safety in terms of atrioesophageal fistula and perhaps slightly better efficacy with respect to AFib burden, but at this point, there are no conclusive data that PFA is superior to thermal or cryoablation from an efficacy or safety standpoint," she adds.
In ADVENT, atrioesophageal fistula and pulmonary vein stenosis did not occur in either group. However, while PFA is thought to avoid collateral heat-related injury, there are some new risks emerging with wider use, including coronary artery spasm and hemolysis.4 Red blood cells are known to be susceptible to electroporation, but the impact of cardiac PFA on erythrocyte destruction is not fully understood.5
"We're excited about [PFA], and we're doing it here, as is everyone else, but it is not a huge game changer as far as I'm concerned, says Kusumoto.
References
- Bunch TJ. Hope, hype, and reality of pulsed field ablation for atrial fibrillation. N Engl J Med 2023;389:1720-21.
- Reddy VY, Gerstenfeld EP, Natale A, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660-71.
- Reddy VY, Mansour M, Calkins H, et al. Pulsed field vs conventional thermal ablation for paroxysmal atrial fibrillation: Recurrent atrial arrhythmia burden. J Am Coll Cardiol 2024;May 18:[Epub ahead of print].
- Gunawardene MA, Schaeffer BN, Jularic M, et al. Coronary spasm during pulsed field ablation of the mitral isthmus line. JACC Clin Electrophysiol 2021;7:1618-1620.
- Popa MA, Bahlke F, Kottmaier M, et al. Myocardial injury and inflammation following pulsed-field ablation and very high-power short-duration ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2024;35:317-27.
Clinical Topics: Arrhythmias and Clinical EP, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Cardiology Magazine, ACC Publications, Atrial Fibrillation, Catheter Ablation, Tachycardia
