Peripheral Matters | Increasing Diversity in Clinical Trials: The ELEGANCE Registry

Lower extremity peripheral artery disease (PAD) is estimated to affect one of 10 Americans aged 40 years or older, with prevalence increasing with age.1,2 Similar patterns are observed worldwide.3
This burden is compounded by significant morbidity and adverse outcomes. PAD causes pain leading to reduced mobility, and severe disease is associated with ischemic wounds and risk of amputation. PAD is also linked with notable health care disparities across sociodemographic groups.
PAD and Health Care Disparities
Inequities associated with PAD presentation, management and outcomes have been extensively described. For example, Black patients in the U.S. experience greater prevalence of severe disease, less robust pharmacological management of risk factors, and increased amputation rates compared with non-Hispanic White patients.4,5
Data on PAD in other race and ethnicity groups are more limited,4 but contributing risk factors such as diabetes and impaired kidney function are highly prevalent among Asian patients, for example, and may impact presentation, disease management and revascularization outcomes.6
Furthermore, women and men experience PAD differently: clinical presentation for women is more likely to be in advanced stages of disease without earlier symptoms, they are less likely to receive guideline-directed therapy compared with men, and women experience more treatment complications.5,7

Despite these disparities, women and patients of racial groups strongly impacted by PAD have been consistently underrepresented in clinical research of PAD treatment. Historical trends persist in recent data showing that female participation in PAD trials is not concordant with disease prevalence.8
Notably, PAD guidelines are based on studies in which only one-third of patients are women and racial minorities comprise only 8%,9 far below the representation of these demographic groups in the population of patients with PAD.
Efforts to Improve Representation in Clinical Trials Initiatives to ensure clinical trial representation consistent with the population intended to be treated are rooted in recognition that sex, race and other characteristics impact health and health care, and further that the entirety of the population affected by a condition deserves care derived from applicable evidence.
Diverse research populations provide generalizable results and intentional inclusion of appropriate groups enables identification of considerations or outcomes unique to those groups.
In the U.S., formal recommendations and policies related to ensuring inclusion of women and minority racial populations trace back to the mid-1980s, but a meaningful impact of prior recommendations and legislation on PAD trials is still to be realized.
Currently, the U.S. Food and Drug Administration and the National Institutes of Health strongly encourage integration of dedicated diversity action plans or inclusion plans as part of trial recruitment and retention plans.11,12
Accountability is achieved through reporting trial data on sex, race and ethnicity. Such plans are developed to facilitate enrollment and retention of a clinically relevant population and perhaps these initiatives will further shift the research landscape.
ELEGANCE Drug-Eluting Registry and Diverse Representation: A Case Study
ELEGANCE is a prospective, multinational, postmarket registry of patients treated with Boston Scientific's drug-eluting devices indicated for the peripheral vasculature.
In order to improve upon sex and race/ethnicity representation in prior PAD studies, ELEGANCE objectives include global enrollment of at least 40% women and 40% of patients from underrepresented racial and ethnic groups.13
The research team has implemented various strategies to enrich the enrolled sample with patients from these populations and recruitment success is evident: among the first 750 enrolled patients, 43% were women and 47% were from underrepresented racial or ethnic groups.13
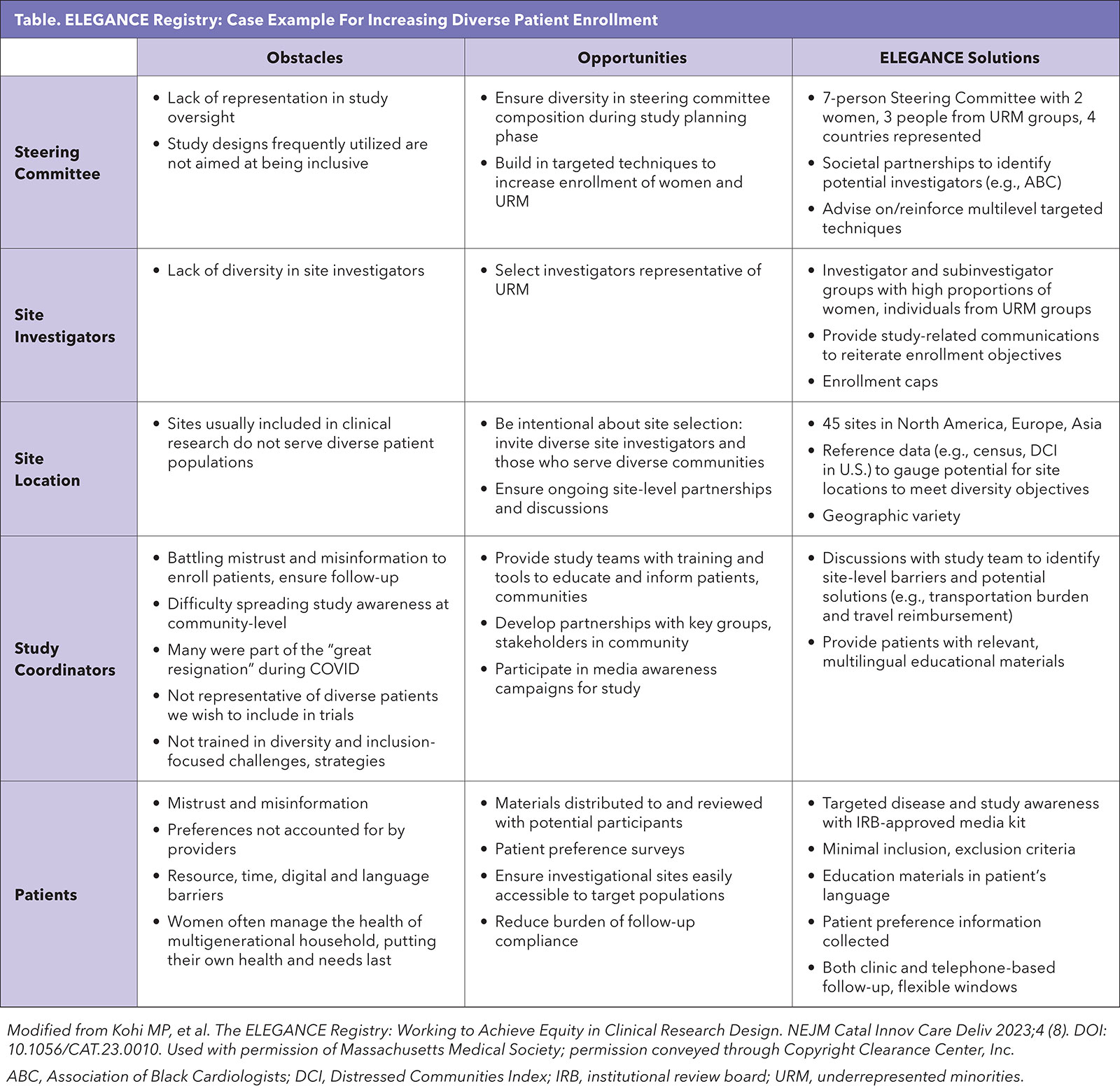
Intentional strategies involving all levels of the research team are needed to execute a multipronged recruitment approach (Table).14
A diverse steering committee provides the foundation for solutions employed in ELEGANCE to achieve enrollment diversity and retain participants. Investigator and site diversity with regard to dimensions beyond research experience or high-enrolling reputation were purposefully sought: investigator gender, race/ethnicity and medical specialty (to account for varied PAD patient referral pathways) were considered, as were site location (urban/rural, geographic region) and other characteristics of the communities served.
The international footprint of the study, with sites currently active in 11 countries, further contributes to representation of diverse patients, health systems and practice patterns. Steering committee and partner (e.g., Association of Black Cardiologists) recommendations, as well as publicly available census and Medicare data and the Distressed Communities Index (DCI)15 were used to target investigator invitations.
Additionally, these data could be referenced in initial discussions between the study team and site investigators to reinforce recruitment expectations.
For example, if a potential study site was located in a community with a low degree of racial or ethnic diversity, strengthening referral pathways might be necessary to reach more diverse patients.
Research teams may consider setting thresholds for relevant sociodemographic characteristics that are available in these types of data sets to aid in identifying study sites.
Instituting enrollment caps is one action taken in ELEGANCE to ensure inclusion of target groups. Specifically, enrollment may be paused or limited at any site with fewer than 40% females or 40% patients from underrepresented racial/ethnic groups at a given milestone. These metrics are closely monitored for each site from the outset of enrollment to ensure recruitment does not skew far from the target.
The study team provides regular communications to remind sites of the enrollment objectives. Underperforming sites may have an incremental target or site-specific "cap" set, necessitating the next enrollment increment (e.g., five patients) to include a certain number of patients from underrepresented groups. The cap remains active until the site is on track. Sites consistently not meeting these objectives have been closed for enrollment.

Study coordinators are at the front line of recruitment activities, positioned to educate and build trust with patients. ELEGANCE study team members meet with site research teams to reiterate study objectives, as well as to learn about enrollment barriers specific to the site.
These discussions in turn empower site personnel to describe the study to patients in a positive manner, and to partner with the study team to address barriers to inclusion.
Investigator and site teams representative of target demographics as well as engaged coordinators all contribute to building patient trust, as does availability of materials in the patient's language.
For ELEGANCE, patient-facing materials including educational pamphlets related to PAD and clinical trials, and informed consent documents are available in multiple languages. These resources further counteract misinformation or lack of patient familiarity with clinical research.
The ELEGANCE team also developed an Institutional Review Board-approved media kit. Such a kit can be provided to community groups to build awareness among their members, or for distribution at a local event, for example. Such outreach leverages existing community connections which a research team may not be equipped to initiate.
ELEGANCE participants have the opportunity to shape the treatment experience for future patient populations. Through the consent process, patients were made aware that "patient preference information" was collected via surveys.
This outcome may be motivating for some patients, as data collected via ELEGANCE patient preference surveys will enhance our understanding of PAD patient values and drivers of decision-making, enabling precision care.
Enrollment diversity is not the end of the story, as targeted patient groups may also have disproportionate attrition. In ELEGANCE, the burden of follow-up compliance is mitigated with remedies including flexible follow-up windows, telephone-based assessments and travel reimbursement (at select sites and in compliance with funding rules).
Clinical research inclusive of the population(s) expected to receive a treatment is essential to yield actionable evidence, and intentional, thoughtful strategies such as those implemented in ELEGANCE are necessary for studies to achieve this goal.
This article was authored by Maureen P. Kohi, MD, principal investigator of ELEGANCE, Department of Radiology, University of North Carolina at Chapel Hill, NC; Jay S. Giri, MD, Associate Fellow of ACC, Department of Cardiovascular Medicine, University of Pennsylvania, Philadelphia; Marianne Brodmann, MD, Division of Angiology, Medical University of Graz, Austria; and Elizabeth J. Davis, PhD, Peripheral Interventions, Boston Scientific, Maple Grove, MN.
References
- Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med 2007;32:328-33.
- Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014;60:686-95 e682.
- Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health 2019;7:e1020-e1030.
- Hackler EL 3rd, Hamburg NM, White Solaru KT. Racial and ethnic disparities in peripheral artery disease. Circ Res 2021;128:1913-26.
- McDermott MM, Ho KJ, Alabi O, et al. Disparities in diagnosis, treatment, and outcomes of peripheral artery disease: JACC Scientific Statement. J Am Coll Cardiol 2023;82:2312-28.
- Chen P, Patel PB, Ding J, et al. Asian race is associated with peripheral arterial disease severity and postoperative outcomes. J Vasc Surg 2023;78:175-183 e173.
- Kavurma MM, Boccanfuso L, Cutmore C, et al. A hidden problem: peripheral artery disease in women. Eur Heart J Qual Care Clin Outcomes 2023;9:342-50.
- Mayor JM, Preventza O, McGinigle K, et al. Persistent under-representation of female patients in United States trials of common vascular diseases from 2008 to 2020. J Vasc Surg 2022;75:30-6.
- Castro-Dominguez Y, Mena-Hurtado C, Algara M, et al. Representativeness of peripheral artery disease randomized clinical trials supporting current guidelines. Am J Cardiol 2023;201:166-9.
- National Academies of Sciences Engineering and Medicine, Policy and Global Affairs, Committee on Women in Science Engineering and Medicine, Committee on Improving the Representation of Women and Underrepresented Minorities in Clinical Trials and Research. Policies to Improve Clinical Trial and Research Diversity: History and Future Directions. In: Bibbins-Domingo K, Helman A, eds. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington DC: National Academies Press; 2022.
- Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies. Draft Guidance for Industry. 2024. Accessed Oct. 25, 2024. Available here.
- Inclusion of Women and Minorities as Participants in Research Involving Human Subjects. 2024. Accessed Oct. 25, 2024. Available here.
- Secemsky EA, Giri J, Brodmann M, et al. Implementing methods in the ELEGANCE registry to increase diversity in clinical research. J Vasc Surg 2024;79:136-45 e133.
- Kohi MP, Secemsky EA, Kirksey L, et al. The ELEGANCE registry: working to achieve equity in clinical research design. NEJM Catalyst Innovations in Care Delivery 2023;4.
- EIG. Distressed Communities Index. 2024. Accessed Nov. 4, 2024. Available here.
Keywords: Cardiology Magazine, ACC Publications, Healthcare Disparities, Diversity, Equity and Inclusion, Clinical Trials as Topic