Advancing the Management of Mitral Regurgitation: A New Era of Personalization and Innovation
Quick Takes
- Artificial intelligence-driven diagnostics, 3D echocardiography, and fusion imaging will enhance mitral regurgitation (MR) assessment and procedural planning.
- Earlier, individualized intervention strategies and combination therapies will redefine MR treatment paradigms.
- Transcatheter therapies (edge-to-edge repair [TEER], transcatheter mitral valve replacement [TMVR]) will expand with newer devices and improved patient selection.
Introduction
Mitral regurgitation (MR) is the most frequently encountered valvular lesion in clinical practice and is associated with significant morbidity and mortality. Previously, surgical mitral valve repair or replacement were the only definitive treatments, providing durable outcomes in appropriately selected patients. Despite advancements in surgical and transcatheter therapies, a substantial proportion of patients with significant MR remain untreated, often due to high surgical risk and delayed recognition. A transformative shift in the management of MR has occurred in the last decade, driven by advances in imaging, medical treatments, and the development of transcatheter therapies.
Diagnostic Advances: Imaging Innovations and Artificial Intelligence
Accurate quantification of MR severity is essential for guiding treatment decisions; however, the recommended multiparameter approach can be time-consuming and requires significant expertise. Multiparameter evaluation may lead to variability in assessments and potential delays in initiating appropriate interventions. With the expansion of transcatheter therapies for MR, more patients—including those previously considered ineligible due to high surgical risk—are now candidates for intervention.
The advent of three-dimensional (3D) echocardiography has significantly enhanced the evaluation by offering detailed visualization of the mitral valve's complex geometry, allowing for more precise assessment of leaflet morphology, annular dimensions, and the spatial orientation of regurgitant jets, thereby enhancing one's understanding of mitral valve anatomy and pathophysiology of MR.1 Left ventricular (LV) assessment in MR should ideally use biplane 2D volumes indexed to body surface area rather than relying solely on linear dimensions, which can significantly underestimate volume changes in asymmetric remodeling. Although 3D echocardiography provides superior accuracy with good correlation to cardiac magnetic resonance (CMR) imaging, it remains underutilized in clinical practice due to dependence on image quality, requirement for specialized expertise, and time constraints in busy clinical settings.2
CMR imaging provides accurate measurements of regurgitant volume and fraction by comparing LV stroke volume with aortic forward flow without geometric assumptions. It can be especially useful in the presence of eccentric jets and offers additional tissue characterization capabilities.3 Despite these advantages, its clinical application remains limited due to availability, cost, and contraindications, making it most valuable as an adjunctive tool when echocardiographic findings are inconclusive or when precise quantification is critical for treatment decisions.
Fusion imaging, integrating 3D echocardiography with computed tomography, is not yet routine but holds promise for preprocedural planning in complex MR interventions, particularly in complex cases and transcatheter mitral valve repair (TMVR).4 Beyond planning, fusion of 3D transesophageal echocardiography with fluoroscopy is improving real-time procedural guidance in transcatheter edge-to-edge repair (TEER) and TMVR, improving device positioning, leaflet grasping, and deployment accuracy (Figure 1).5 This technique enhances visualization of soft tissue structures while reducing fluoroscopy reliance. As these technologies advance, fusion imaging will likely play an increasing role in both preprocedural assessment and interventions.
Figure 1: Fusion Imaging Techniques for Procedural Planning and Guiding of Transcatheter Mitral Regurgitation Treatments
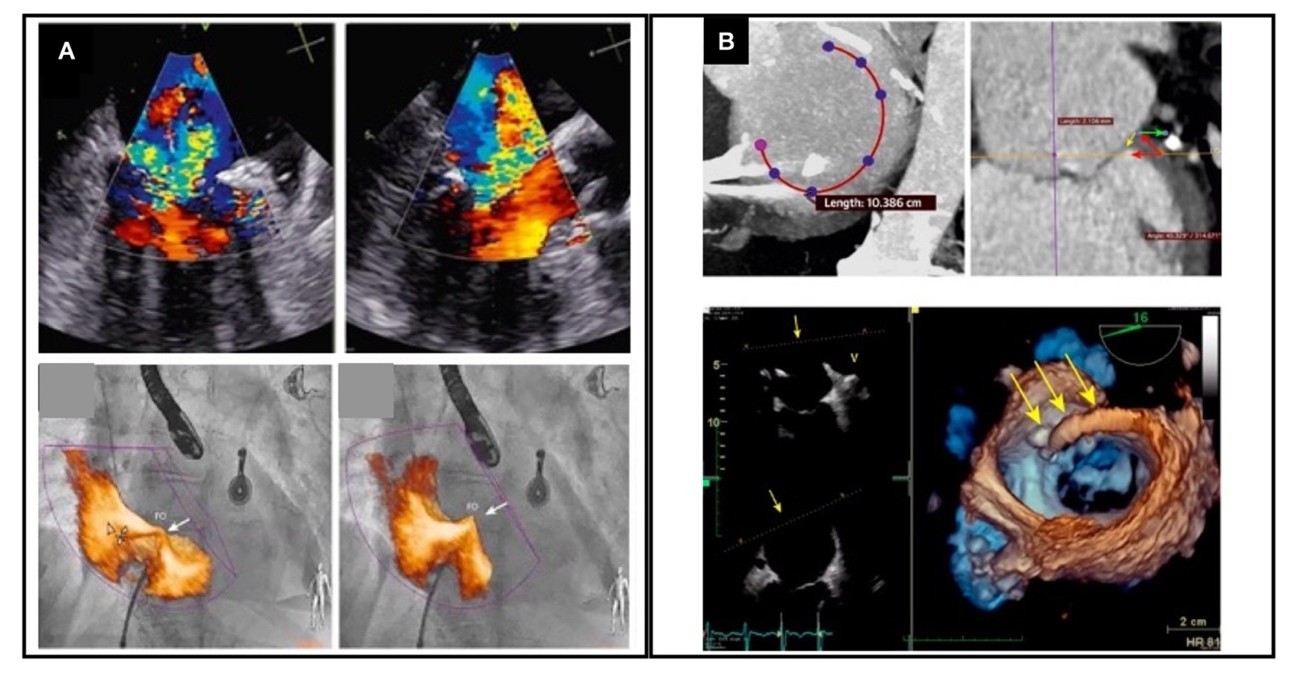
Modified with permission from Bax JJ, Debonnaire P, Lancellotti P, et al. Transcatheter interventions for mitral regurgitation: multimodality imaging for patient selection and procedural guidance. JACC Cardiovasc Imaging. 2019;12(10):2029-2048. doi:10.1016/j.jcmg.2019.03.036.
Panel A shows intraprocedural fusion imaging using transesophageal echocardiography (TEE) and fluoroscopy during a transcatheter edge-to-edge procedure. Panel B shows fusion imaging of three-dimensional TEE and computed tomography for preprocedural planning of a cardioband.
Recently, artificial intelligence (AI) and deep learning models have been developed to assist in the diagnosis and quantification of MR, as well as to predict outcomes following interventions. Advanced algorithms can analyze large datasets from echocardiographic studies to identify patterns indicative of MR severity, potentially surpassing standard diagnostic capabilities. Recent studies have developed algorithms that accurately grade the severity of MR from color Doppler videos, as well as by using multiparametric approaches to predict patient outcomes after MR interventions by integrating imaging data with clinical parameters, facilitating personalized treatment planning and risk stratification.6,7
Expanding Treatment Indications and Personalized Approaches
The indications for treating primary MR (PMR) are well defined, based on the presence of symptoms, LV size, and systolic function. However, ongoing randomized controlled trials aim to refine the optimal timing for intervention in asymptomatic PMR patients, particularly in those with early signs of LV enlargement, subclinical myocardial dysfunction, assessed by strain imaging, or atrial fibrillation onset (Dutch-AMR [Early Mitral Valve Repair Versus Watchful Waiting in Asymptomatic Patients With Severe Mitral Regurgitation] trial).
The management of secondary mitral regurgitation (SMR) or functional MR (FMR) is complex and subject to ongoing refinement as new evidence and therapeutic strategies emerge. The COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) and MITRA-FR (Percutaneous Repair With the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation) trials provided conflicting results regarding the benefit of TEER in SMR, emphasizing the critical role of patient selection.8 Although both studies evaluated the use of MitraClip™ in patients with severe SMR, key differences in their patient populations likely contributed to their divergent outcomes. COAPT enrolled patients with severe MR but relatively preserved LV size, suggesting that MR itself was a major driver of heart failure (HF) symptoms. In contrast, MITRA-FR included patients with more dilated LV and lower ejection fractions (EFs), whereas MR was likely a secondary consequence of global ventricular dysfunction rather than the primary pathology.
These differences highlight the concept of proportionate versus disproportionate MR, which plays a crucial role in treatment selection. In proportionate MR, the degree of regurgitation is in line with the severity of LV dilation and dysfunction, making the underlying cardiomyopathy the primary therapeutic target. These patients typically benefit from optimized guideline-directed medical therapy (GDMT), volume management, cardiac resynchronization therapy in the presence of conduction delay, and atrial fibrillation ablation if indicated. Conversely, in disproportionate MR, the severity of regurgitation exceeds what would be expected given the degree of LV dysfunction, suggesting that MR itself is a primary driver of HF progression. In these patients, targeting the valvular pathology should be the key therapeutic priority.
MR in patients with HF with preserved EF (HFpEF) is typically secondary, often resulting from left atrial dilatation (atrial functional MR, aSMR) rather than an intrinsic mitral valve pathology. Real-world registry data support the similar procedural success rates and clinical outcomes of using TEER in patients with HFpEF in aSMR compared with ventricular SMR.9
Advances in Transcatheter Treatments
The evolution of transcatheter therapies has significantly expanded treatment options for both PMR and SMR. TEER remains the most widely established transcatheter intervention, with next-generation devices improving procedural efficiency and outcomes. The MitraClip system has undergone iterative enhancements, including independent leaflet grasping and expanded device sizes, broadening its applicability to a wider range of anatomical variations. The PASCAL system, featuring wider paddles and a novel grasping mechanism, offers an alternative approach that may improve MR reduction in select patients (Figure 2).
Figure 2: Transcatheter Edge-to-Edge Repair Systems (MitraClip and PASCAL)
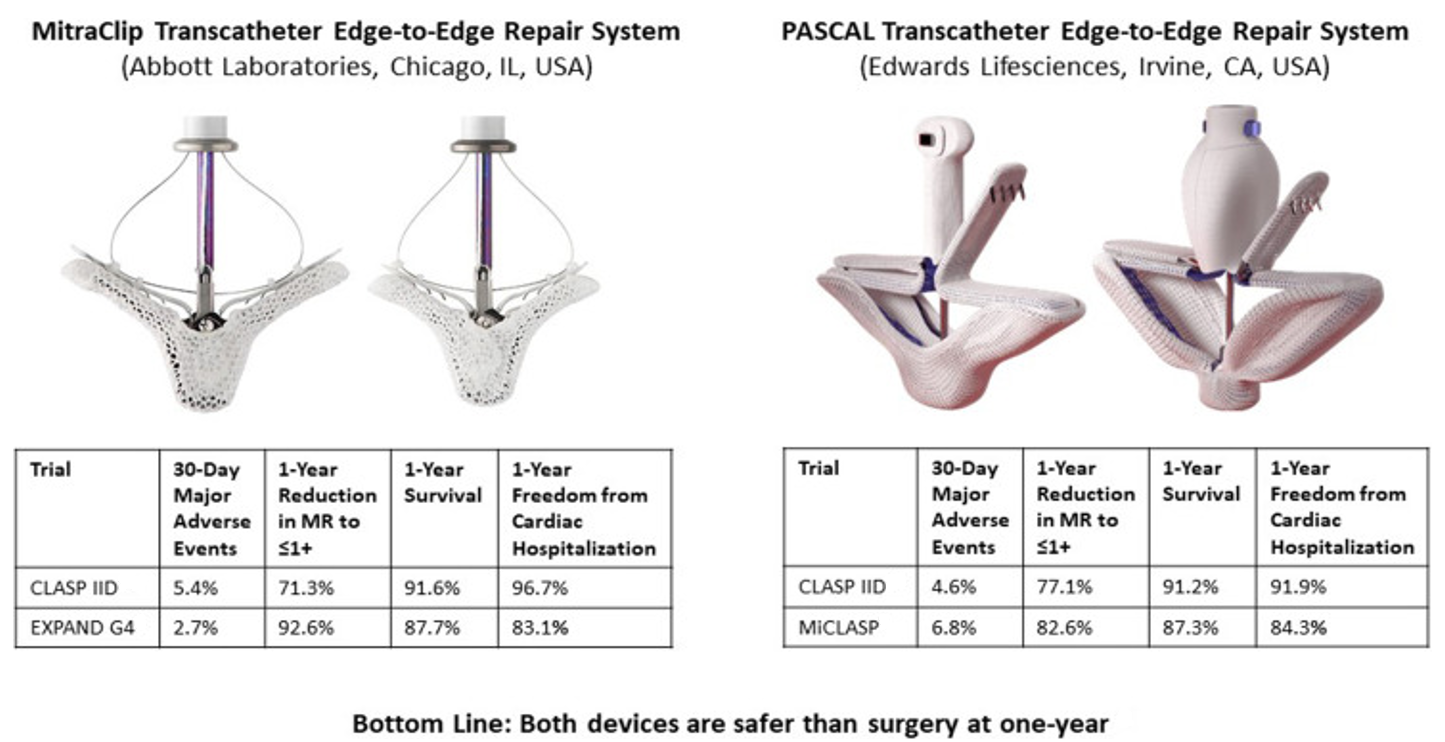
Reprinted with permission from Donal E, Gegout L, Leurent G, Lee KC. Transcatheter edge-to-edge repair of mitral regurgitation: a mature technique. JACC Cardiovasc Interv. 2024;17(7):904-906. doi:10.1016/j.jcin.2024.03.008.
Beyond TEER, TMVR is emerging as a promising alternative for patients who are unsuitable for valve repair due to leaflet tethering, annular calcification, or extensive mitral pathology. Unlike TEER, which primarily reduces regurgitant volume, TMVR aims to fully eliminate MR. However, its adoption has been limited by challenges such as LV outflow tract obstruction (LVOTO), device anchoring, and valve durability. Several TMVR systems are currently being evaluated, including the Intrepid™ valve (APOLLO [Transcatheter Mitral Valve Replacement With the Medtronic Intrepid™ TMVR System in Patients With Severe Symptomatic Mitral Regurgitation] trial) and the Tendyne valve (SUMMIT [Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Transcatheter Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation] trial), which has already received CE approval in Europe (January 2020). Additional transapical and trans-septal TMVR platforms are shown in Figure 3.
Figure 3: Transapical and Trans-Septal Systems for Transcatheter Mitral Valve Replacement
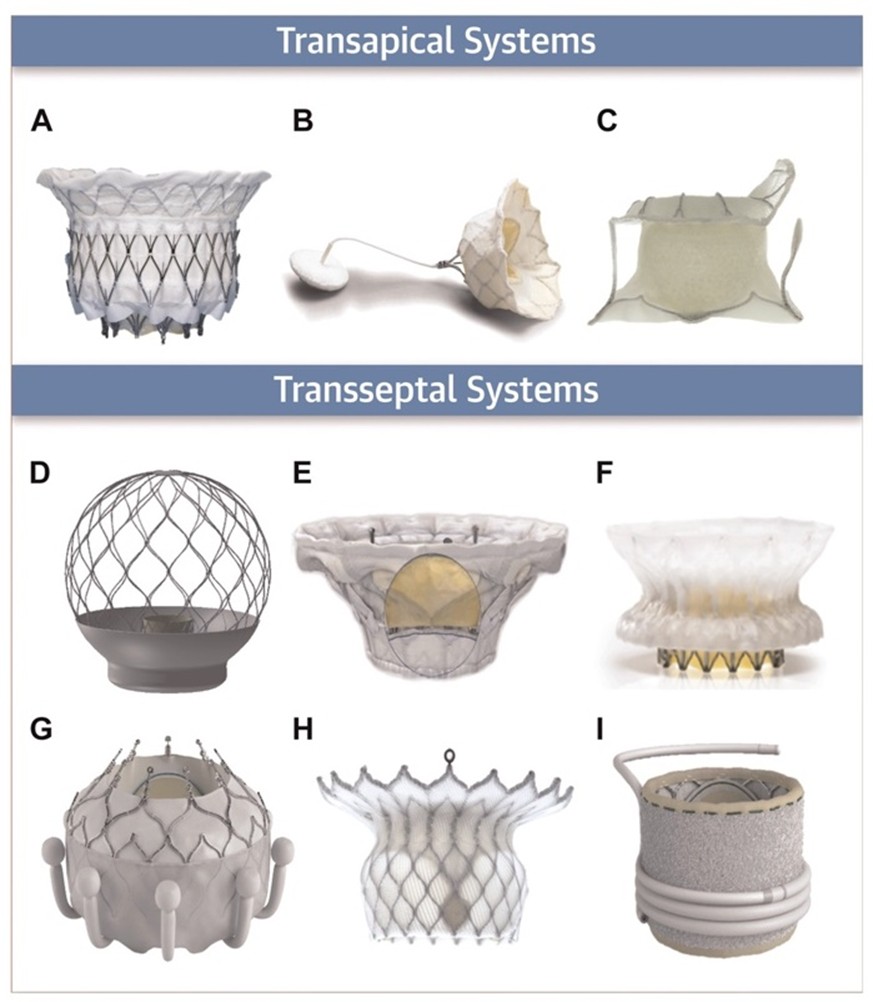
Reprinted with permission from Hensey M, Brown RA, Lal S, et al. Transcatheter mitral valve replacement: an update on current techniques, technologies, and future directions. JACC Cardiovasc Interv. 2021;14(5):489-500. doi:10.1016/j.jcin.2020.12.038.
Transapical valves: (panel A) Intrepid™, (panel B) Tendyne, and (panel C) Tiara. Trans-septal valves: (panel D) AltaValve, (panel E) Cardiovalve, (panel F) Cephea, (panel G) EVOQUE, (panel H) HighLife, and (panel I) SAPIEN M3. (Panel A) Image courtesy of Medtronic. (Panels B, F) Images courtesy of Abbott. (Panel C) Image courtesy of NeoVasc. (Panel D) Image courtesy of 4C Medical. (Panel E) Image courtesy of Cardiovalve. (Panels G, I) Images courtesy of Edwards Lifesciences. (Panel H) Image courtesy of HighLife Medical.
Annuloplasty-based interventions are also gaining traction as adjunctive or stand-alone therapies for MR. The Carillon® Mitral Contour System, a coronary sinus-based annuloplasty device, indirectly reduces annular dilation, whereas the AccuCinch system is designed to restore LV geometry, potentially altering the disease course in SMR patients (Figure 1). These approaches may serve as bridge therapies or complementary interventions alongside TEER or TMVR, particularly for patients with significant annular dilation.
Ongoing randomized controlled trials will provide critical insights into patient selection, optimal timing of intervention, and the durability of transcatheter approaches. The REPAIR MR (Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients With Primary Mitral Regurgitation Who Are Candidates for Surgery) trial is currently investigating TEER for primary MR, particularly exploring feasibility in patients with moderate surgical risk. The results from the RESHAPE-HF2 (Randomized Investigation of the MitraClip Device in Heart Failure: Second Trial in Patients With Clinically Significant Functional Mitral Regurgitation) trial recently showed that TEER is feasible in patients with HF and reduces cardiovascular death and HF hospitalizations compared with GDMT alone.10 Such studies can help refine treatment algorithms and guideline recommendations, ensuring more personalized and evidence-based MR management.
Conclusion and Future Outlook
Management of MR is progressing due to advances in transcatheter therapies, AI-driven diagnostics, and earlier intervention strategies. As TEER and TMVR technologies evolve, patient selection will become increasingly refined, enabling more effective and durable treatment options.
In the coming years, MR guidelines are likely to shift toward more proactive and individualized treatment, perhaps emphasizing earlier percutaneous intervention (Figure 4; Table 1).
Figure 4: Overview of the Most Relevant Developments on a Timeline of the Past, Present, and Future Era of Management of Mitral Regurgitation
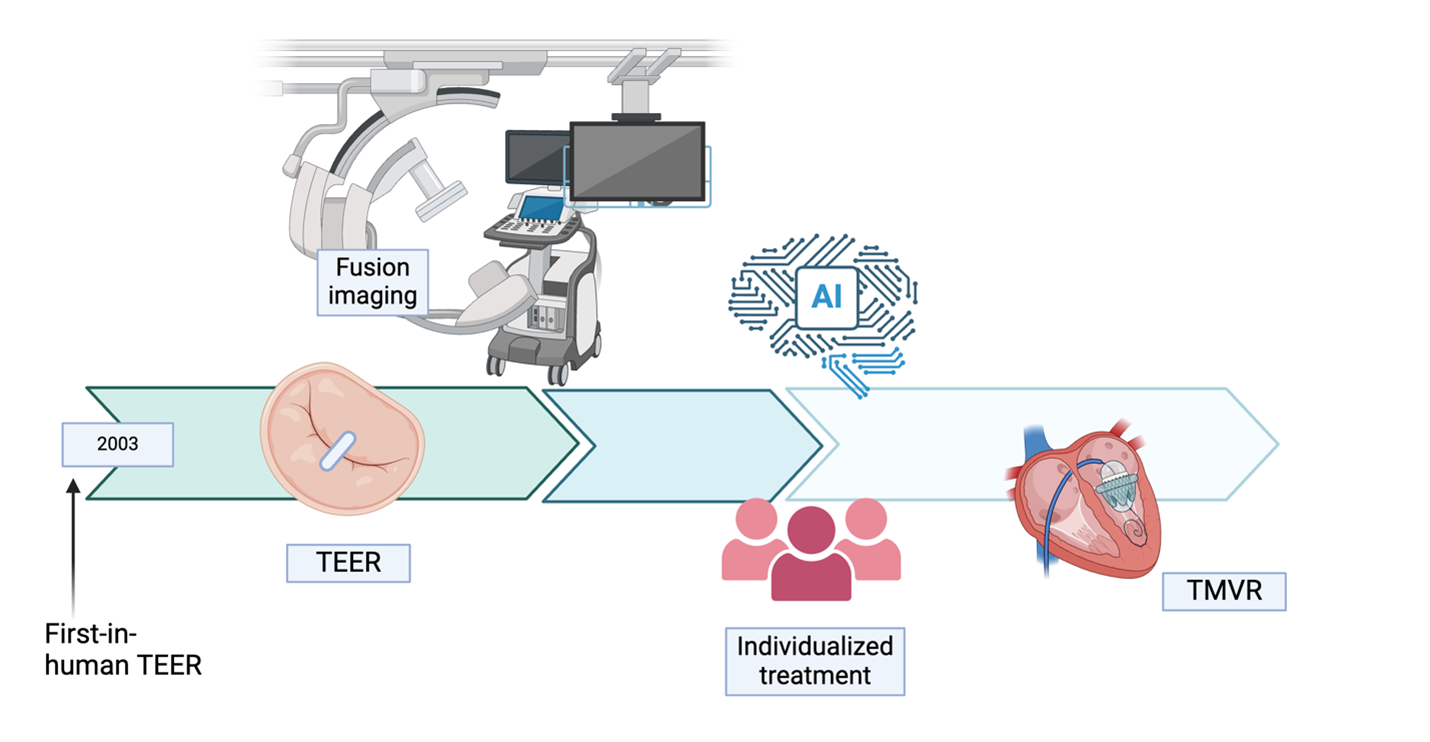
Figure was created in BioRender. Binder-Rodriguez C.(2025) https://BioRender.com/bgz90d2.
AI = artificial intelligence; TEER = transcatheter edge-to-edge repair; TMVR = transcatheter mitral valve replacement.
Table 1: Overview of Published and Ongoing Randomized Controlled Trials Evaluating Safety and Efficacy of Transcatheter Therapies for Mitral Valve Regurgitation
| Trial | Year (clinicaltrials.gov ID) | Population | Intervention | Duration | Key Findings and Primary Endpoints |
| Transcatheter Edge-to-Edge Repair (TEER) | |||||
| EVEREST II | 2013 (NCT01940120) |
Symptomatic FMR and DMR, LVEF ≥25%, LVESD ≤55 mm | TEER (MitraClip™) vs. Surgery | Up to 5-year follow-up |
|
| COAPT | 2018 (NCT01626079) |
Severe SMR, LVEF 20-50%, LVEDVi <96 mL/m2 | TEER (MitraClip) + GDMT vs. GDMT alone | Up to 5-year follow-up |
|
| MITRA-FR | 2018 (NCT01920698) |
Severe SMR, LVEF <50%, LVEDVi >96 mL/m2 | TEER (MitraClip) + GDMT vs. GDMT alone | Up to 2-year follow-up |
|
| CLASP IID | 2022, 2023 (NCT03706833) |
DMR with prohibitive surgical risk and FMR on GDMT | TEER (PASCAL) vs. MitraClip | Up to 5-year follow-up |
|
| EXPAND | 2023 (NCT03502811) |
PMR and SMR |
TEER (MitraClip NTR/XTR and G4) | Up to 1-year follow-up |
|
| MATTERHORN | 2024 (NCT02371512) |
HF and SMR, mean LVEF ≥20% | TEER (MitraClip) vs. surgical MVR | 1 year |
|
| RESHAPE-HF | Terminated due to low recruitment rate (NCT01772108) |
SMR, HFrEF (LVEF 15-40% and NYHA class III or IV) | TEER (MitraClip) vs. GDMT | 2 years |
|
| RESHAPE-HF 2 | 2024 (NCT02444338) |
FMR, HF (LVEF 20-50% and NYHA class II or IV) | TEER (MitraClip) vs. GDMT | 2 years |
|
| REPAIR MR | Ongoing (NCT04198870) |
Severe PMR, moderate surgical risk, unsuitable for surgical MVR | TEER (MitraClip) vs. late surgery | 2 years |
|
| CLASP IID/IIF | Ongoing (NCT03706833) |
DMR with prohibitive surgical risk and FMR on GDMT | TEER (PASCAL) vs. MitraClip | Up to 5-year follow-up |
|
| Transcatheter Mitral Valve Replacement (TMVR) | |||||
| SUMMIT | Ongoing (NCT03433274) |
Symptomatic, at least moderate-to-severe MR | Tendyne™ valve vs. MitraClip | 1 year |
|
| APOLLO | Ongoing (NCT03242642) |
Severe symptomatic MR | Intrepid™ valve vs. GDMT | 1 year |
|
| MICEND | Ongoing (NCT02718001) |
Significant MR, high risk for surgical MVR | EVOQUE Eos MV | 30 days (up to 5-year follow-up) |
|
| TIARA-II | Ongoing (NCT03039855) |
Severe MR, high risk for surgical MVR | Tiara™ | 30 days (up to 5-year follow-up) |
|
| AltaValve | Ongoing (NCT06465745) |
At least moderate-to-severe MR, unsuitable for surgical MVR or TEER | AltaValve™ | Up to 1-year follow-up |
|
| ENCIRCLE | Ongoing (NCT04153292) |
At least moderate-to-severe MR, unsuitable for surgical MVR or TEER | Sapien M3 | 1 year |
|
| HighFLO | Ongoing (NCT04888247) |
At least moderate-to-severe MR, unsuitable for surgical MVR, anatomically at risk for LVOTO | HighLife CLARITY | 24 hours |
|
| AHEAD | Ongoing (NCT03339115) |
At least moderate-to-severe MR, unsuitable for surgical MVR | Cardiovalve® | Up to 2-year follow-up |
|
| Cephea early feasibility study | Ongoing (NCT05061004) |
At least moderate-to-severe MR and/or severe MS | Cephea | 30 days |
|
| Interventional Annuloplasty | |||||
| ACTIVE | Terminated (NCT03016975) |
Significant FMR and symptomatic HF | Cardioband vs. GDMT | 1 year |
|
| REDUCE-FMR | 2019 (NCT02325830) |
At least moderate FMR and symptomatic HF | Carillon® vs. GDMT | Up to 1-year follow-up |
|
| EMPOWER | Ongoing (NCT03142152) |
HF and at least mild FMR | Carillon vs. GDMT | Up to 2-year follow-up |
|
| CorCinch-FMR | Completed, results pending (NCT02806570) |
HF and at least moderate FMR | AccuCinch® | 30 days |
|
AE = adverse event; AKI = acute kidney injury; DMR = degenerative mitral regurgitation; FDA = Food and Drug Administration; FMR = functional mitral regurgitation; GDMT = guideline-directed medical therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LV = left ventricle; LVEDVi = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESD = left ventricular systolic diameter; LVOTO = left ventricular outflow tract obstruction; MR = mitral regurgitation; MS = mitral stenosis; MVR = mitral valve repair; NYHA = New York Heart Association; PMR = primary mitral regurgitation; SMR = secondary mitral regurgitation; TEER = transcatheter edge-to-edge repair.
References
- Harm T, Schwarz FJ, Zdanyte M, et al. Novel 3-dimensional effective regurgitation orifice area quantification serves as a reliable tool to identify severe mitral valve regurgitation. Sci Rep. 2024;14(1):22067. Published 2024 Sep 27. doi:10.1038/s41598-024-73264-4
- Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;59(20):1799-1808. doi:10.1016/j.jacc.2012.01.037
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. doi:10.1016/j.echo.2017.01.007
- Man JP, Bouma BJ, Schuuring MJ. Fusion imaging in preoperative planning of mitral valve surgery to prevent injury of the left circumflex artery. Eur Heart J. 2022;43(45):4762. doi:10.1093/eurheartj/ehac566
- Faletra FF, Pozzoli A, Agricola E, et al. Echocardiographic-fluoroscopic fusion imaging for transcatheter mitral valve repair guidance. Eur Heart J Cardiovasc Imaging. 2018;19(7):715-726. doi:10.1093/ehjci/jey067
- Sadeghpour A, Jiang Z, Hummel YM, et al. An automated machine learning-based quantitative multiparametric approach for mitral regurgitation severity grading. JACC Cardiovasc Imaging. 2025;18(1):1-12. doi:10.1016/j.jcmg.2024.06.011
- Vrudhula A, Duffy G, Vukadinovic M, Liang D, Cheng S, Ouyang D. High-throughput deep learning detection of mitral regurgitation. Circulation. 2024;150(12):923-933. doi:10.1161/CIRCULATIONAHA.124.069047
- Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20(6):620-624. doi:10.1093/ehjci/jez073
- Masiero G, Montonati C, Rubbio AP, et al. Impact of transcatheter edge-to-edge mitral valve repair on atrial functional mitral regurgitation from the GIOTTO registry. Am J Cardiol. 2024;211:219-227. doi:10.1016/j.amjcard.2023.11.007
- Anker SD, Friede T, von Bardeleben RS, et al. Transcatheter valve repair in heart failure with moderate to severe mitral regurgitation. N Engl J Med. 2024;391(19):1799-1809. doi:10.1056/NEJMoa2314328
Clinical Topics: Valvular Heart Disease, Mitral Regurgitation, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging
Keywords: Mitral Valve Insufficiency, Regurgitation, Mitral Valve, Artificial Intelligence