Cover Story | Interventional Management in Acute Type B and Type A Aortic Dissection
Acute aortic dissection may be life-threatening and initial therapy includes stabilization, anti-impulse blood pressure control with beta-blocker, urgent surgery for Type A (ascending – proximal to the brachiocephalic artery) dissection and optimal medical therapy (and intervention for complications) for Type B (not involving the ascending aorta, typically distal to the left subclavian artery) dissection. Optimal medical therapy includes reducing the systolic blood pressure to <120 mm Hg and heart rate to <70 beats/minute.
Individuals with an acute Type B aortic dissection (TBAD) have an in-hospital mortality rate of about 10%, but for initially uncomplicated TBAD, those treated medically have a 1-6% in-hospital mortality.1-4
Complicated TBAD occurs in 25-30% of patients, and malperfusion, aortic rupture, acute aortic expansion and refractory pain are associated with a high morbidity and mortality.1,5
Thoracic endovascular aortic repair (TEVAR) is the preferred treatment for acute complicated TBAD and may correct malperfusion and treat enlarging aneurysms and acute rupture, with open surgery reserved for those not treatable by endovascular techniques.6
Data from the International Registry of Acute Aortic Dissection (IRAD) reveals the treatment pattern for TBAD: 57% of patients were treated medically, 32% with endovascular repair and 7% with open surgery with mortality rates of 10%, 14% and 21% for each group, respectively.1 Other series report higher mortality with open surgical repair.7
Patients with initially uncomplicated TBAD are at risk for progressive aneurysmal dilatation of the aorta (occurring in at least 30-40%) and late aortic rupture.5 Certain imaging characteristics may predict a higher risk for aortic complications during follow-up.5
TEVAR, by covering the primary tear and directing the blood flow into the true lumen, promotes false lumen thrombosis, slows dilatation of the downstream aorta and allows aortic remodeling.
TEVAR for uncomplicated TBAD holds promise to improve long-term aortic outcomes and is the subject of international investigation.
This article reviews endovascular treatment in acute aortic dissection discussing its role primarily in TBAD and introduces its limited experience in Type A dissection (TAAD).
Interventional Management of Acute Complicated TBAD
Patients who suffer with TBAD typically require intervention if the presentation is associated with one of the following complicating features:
- impending or frank rupture,
- end-organ malperfusion,
- rapidly expanding false lumen (>5 mm on short interval imaging),
- persistent pain refractory to medical therapy.
At least 25% of initially uncomplicated TBAD progress to a complicated state.8 Rupture, occurring in <5% of TBAD, is characterized by periaortic hematoma or hemorrhagic pleural effusion on computerized tomography imaging, often with hemodynamic instability.
Malperfusion syndromes arise from false lumen extension with either dynamic or static compromise of flow into abdominal or thoracic aortic branches. This may manifest as spinal cord ischemia, renal or visceral ischemia, or lower extremity ischemia.
Endovascular Stenting
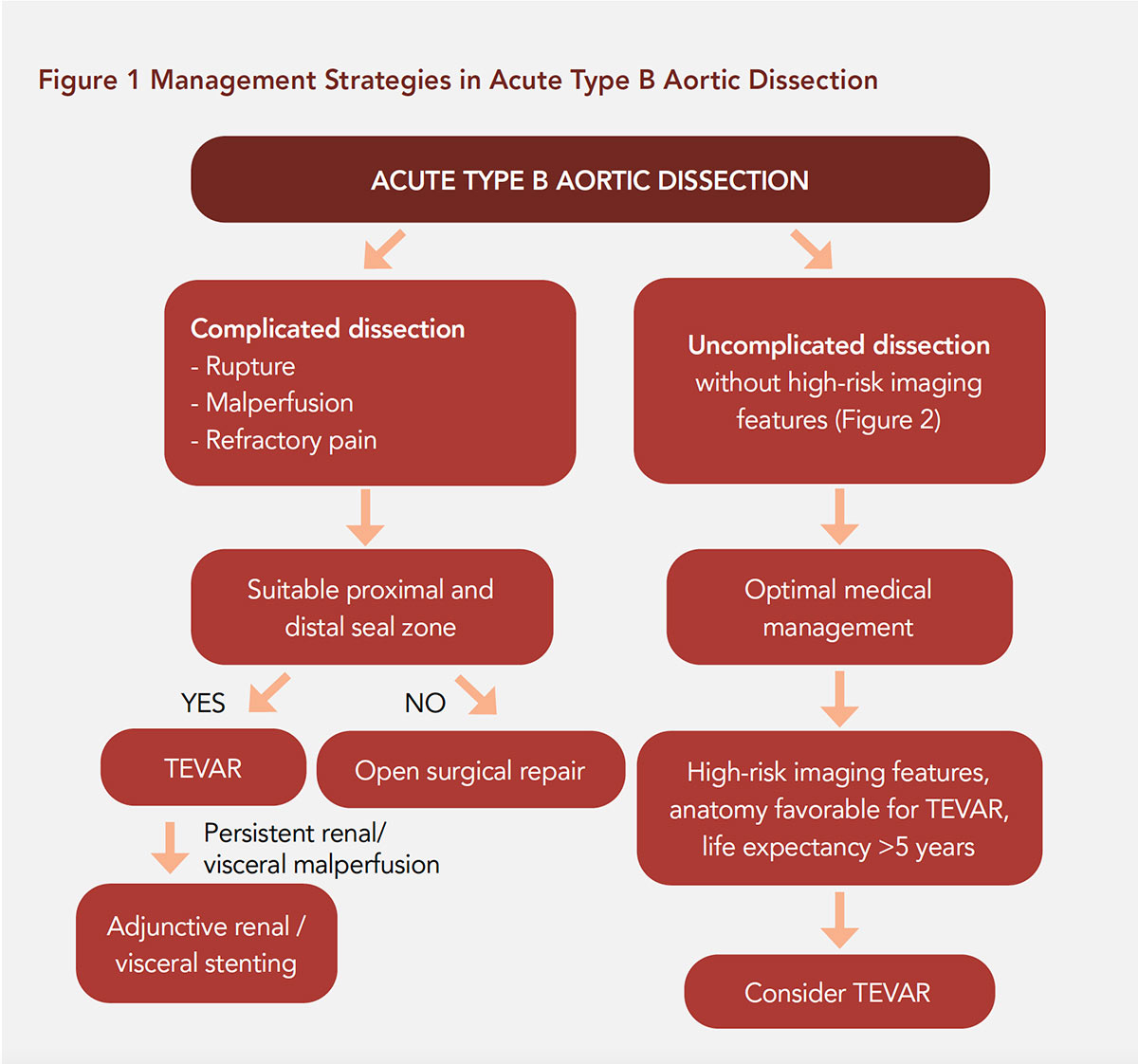
Endovascular stenting of the descending aorta has evolved as the first-choice treatment option for complicated TBAD (Figure 1).2, 9-12 The "early" time period for TBAD refers to the period ≤90 days from onset of symptoms, encompassing the hyperacute (<24 hours), acute (1-14 days) and subacute (15-90 days) periods.2
Complicated TBAD most often develops in the hyperacute or acute time periods and comprise roughly one-quarter of all patients presenting with acute dissection.
Several endovascular techniques are available to treat complicated TBAD, including TEVAR, branch vessel stenting for residual compromised flow, and least commonly balloon fenestration of the dissection septum if primary tear coverage is not anatomically feasible.
TEVAR for complicated TBAD has led to less morbidity and mortality when compared to open surgical results.13-15 While this endovascular strategy now represents the current preferred first-line therapy for complicated TBAD, several key considerations remain unresolved.
Specifically, the optimal timing, aortic endograft sizing (proximal and distal), stent-graft type, coverage length and role of adjunctive false lumen therapies remain poorly defined. In addition, the development of novel technologies and thresholds for concomitant visceral-renal stenting has evolved.
The rationale for and timing of TEVAR in TBAD generally incorporate the following considerations:
- duration of time from symptom onset,
- location of the proximal entry tear,
- aortic dimensions in the proximal and distal landing zones,
- segmental anatomic extent involved in the dissection,
- associated complications from the dissection (e.g., rupture vs. malperfusion),
- false lumen (FL) status.
Practical Strategies in TEVAR
Appropriate proximal landing zone anatomy for TEVAR is typically based on non-dissected aorta (between left common carotid-left subclavian artery) inner (intima-intima) diameter ≤42 mm and a centerline distance from the distal edge of the left common carotid artery to the proximal primary entry tear ≥20 mm.
Recommended aortic endograft sizing is typically 0-10% in the proximal landing zone, to avoid risk of retrograde TAAD. IVUS is strongly recommended to verify true lumen (TL) anatomy, landing zone dimensions and branch-vessel morphology.
Distal oversizing of the aortic endograft is more forgiving, but generally not recommended to exceed 10-20% of the maximal transaortic diameter within the targeted distal landing zone.
In cases of rupture, aortic coverage should extend from the proximal entry tear to the celiac artery origin, with the primary objective to exclude FL flow. In contrast, when malperfusion is the primary complicating feature, the optimal strategy for length of aortic coverage is less well established.
Recently, series describing more aggressive coverage length strategies (e.g., >220-240 mm) report improved long-term aortic remodeling outcomes and decreased re-intervention without elevated spinal cord ischemia rates.10, 11 IVUS is very helpful in these scenarios, as short segment endograft (~15 cm) can be deployed for primary entry tear coverage.
If afterwards IVUS reveals FL depressurization and TL expansion, with brisk visceral vessel perfusion confirmed by angiography, then consideration can be made to follow the patient clinically without extending the endograft to level of the celiac artery.
If post-deployment of a shorter segment endograft does not achieve satisfactory TL expansion, an endograft extension can be performed. If malperfusion is not relieved with TEVAR alone, additional endovascular treatment of persistent dynamic or static obstruction of the distal branch vessels may be required with branch vessel stenting.
Self-expanding stents are favored to reduce flap mobility within the branch lumen. Balloon-expandable stents are generally avoided due to concern for compression by the residual dissection flap. Compliant balloon molding of the endograft in the acute setting is typically avoided unless there is evidence of endoleak and/or significant persistent FL perfusion in cases of rupture.
This practice mitigates risk of retrograde TAAD or new stent-induced entry (SINE) tear. In cases requiring left subclavian coverage, concomitant revascularization is strongly recommended except in salvage cases due to frank rupture.12
Retrograde Dissection
Certain independent predictors of retrograde dissection have been identified which are not modifiable and include preoperative evidence of FL aneurysm enlargement, visceral or mesenteric malperfusion or COPD.
However, other factors such as >10% over sizing, compliant ballooning, associated intramural hematoma (IMH) in the transverse arch, and bare stent in the proximal endograft have been found to be associated with retrograde dissection.13,14
If there is extensive IMH through the aortic arch, consideration should be given to an alternative surgical approach. This scenario frequently requires aortic arch replacement via median sternotomy, with a possible frozen elephant trunk procedure where an endograft is deployed in antegrade fashion into the descending thoracic aorta.
Data regarding use of endografts with an uncovered proximal bare stent and risk of retrograde dissection are mixed, however many centers favor dissection-specific devices without proximal bare stent configuration.15
The Achilles Heel
A re-intervention rate of 15-30% following TEVAR for acute complicated TBAD is the Achilles heel, and has led to innovative endograft designs to address specific anatomic challenges associated with endovascular treatment of TBAD. One potential solution is the combination of a proximal covered stent and distal bare stent (e.g., provisional extension to induce complete attachment or PETTICOAT technique).11
While this strategy does not cover any distal re-entry tears, the distinct advantage of this technique is to help mitigate spinal cord ischemia and perhaps the need for visceral branch stenting.16,17
Endovascular Therapy in Initially Uncomplicated TBAD
Optimal medical therapy has been the cornerstone of treatment for uncomplicated TBAD, being associated with relatively low in-hospital mortality rates ranging from 1-10%.1,4
The term "uncomplicated" refers to aortic in-hospital mortality and morbidity. Nevertheless, despite adequate pharmacological and lifestyle modification, aortic-related complications will appear in 20-50% of patients within four to seven years from an acute event.18-20
Because of the high-risk for late aortic events in TBAD, interest in "prophylactic" TEVAR for uncomplicated TBAD has been investigated in many cardiovascular centers worldwide and in randomized trials including INSTEAD, INSTEAD XL and ADSORB comparing TEVAR to optimal medical therapy.21-23
The INSTEAD trial reported similar mortality rates at two years when comparing TEVAR to medical therapy in the subacute/chronic phase, but improved aortic remodeling, including FL thrombosis and TL expansion.21
By five years, those receiving TEVAR had improved aortic-related mortality and remodeling outcomes.22 ADSORB found TEVAR to improve aortic remodeling, but no mortality difference at one year.23
TEVAR for uncomplicated TBAD carries risks including retrograde dissection, stroke, spinal cord ischemia, post-implantation syndrome and continued perigraft flow.
When considering initially uncomplicated TBAD, there are important chronological, clinical and anatomical issues to consider when weighing the risks and benefits of endovascular treatment.
Chronological Considerations
TEVAR as an alternative therapy to optimal medical therapy in uncomplicated TBAD should evaluate the concept of "time until treatment equipoise" (TUTE) that has been described in an attempt to clarify patients of relative risks of different management modalities.24
TUTE is the point in time during follow-up after which an intervention is most beneficial, because the mortality risk of the intervention is lower than the mortality risk of continuing current medical management.
The early hazard of performing endovascular therapy must be offset by the ability of TEVAR to prevent aneurysm formation (and requirement for open or other therapy) in the future. The equipoise is set at the point where the areas between the survival curves of no intervention and intervention are equal; for uncomplicated TBAD this is not yet well defined.
The timing of prophylactic aortic intervention is also important in treatment outcomes. While early interventions may provide advantages for aortic remodeling (when the dissection flap is most pliable), complications including retrograde dissection and SINE may be more common immediately after the acute dissection.25
In the INSTEAD trial, patients were enrolled a median of 39 days after the dissection and underwent TEVAR at a median of 12 days after randomization.21
In the VIRTUE registry, TEVAR in the subacute setting for complicated TBAD suggests that aortic "plasticity" persists for several weeks after acute dissection and interventions in the subacute interval associate with favorable outcome and low rate of acute complications.26 Waiting at least two weeks before elective TEVAR in TBAD when performed for aortic remodeling has been recommended.25
Clinical Considerations
The age of patients seems to affect aortic volume increase during follow-up. Female patients younger than 60 years have shown a significantly increased aortic growth rate, while a decreased growth rate has been associated with increasing age.27,28
Blood pressure control, smoking cessation and adherence to a multidrug regimen including beta-blockers are important in long-term management after aortic dissection.29
In nonrandomized studies, calcium channel blockers and ACE inhibitors associate with fewer aortic events and improved outcomes.1
Laboratory findings such as fibrinogen-fibrin degradation product (FDP) level >20 mg/mL at admission has been associated with aortic enlargement within three months.30
TEVAR for uncomplicated TBAD is not recommended for individuals with heritable thoracic aortic aneurysm disease (i.e., Marfan syndrome, Loeys-Dietz syndrome), due to an increased risk of adverse outcomes and re-interventions.31
Anatomical Considerations
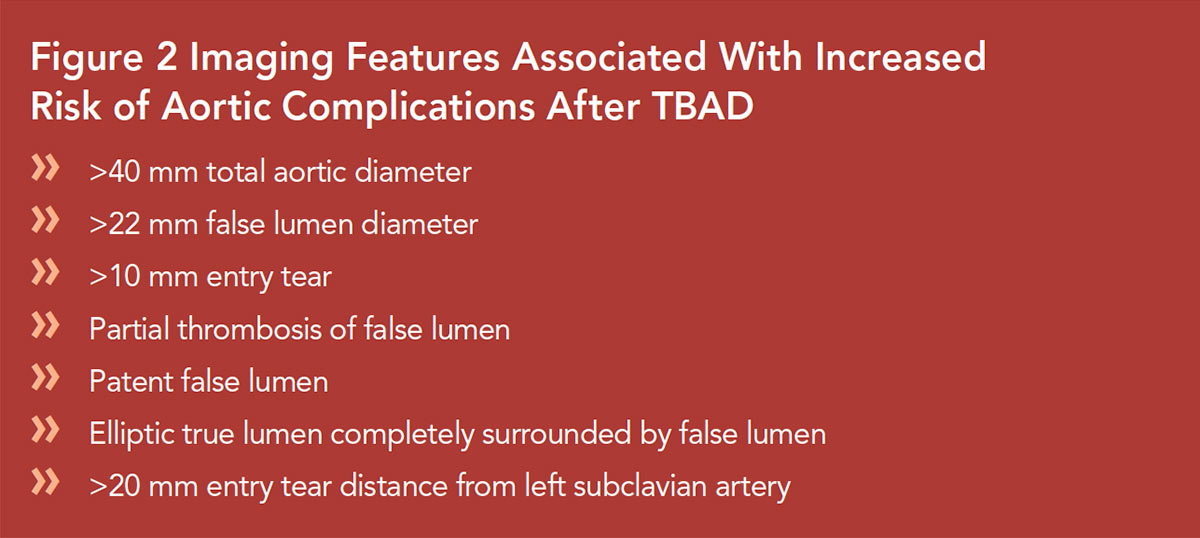
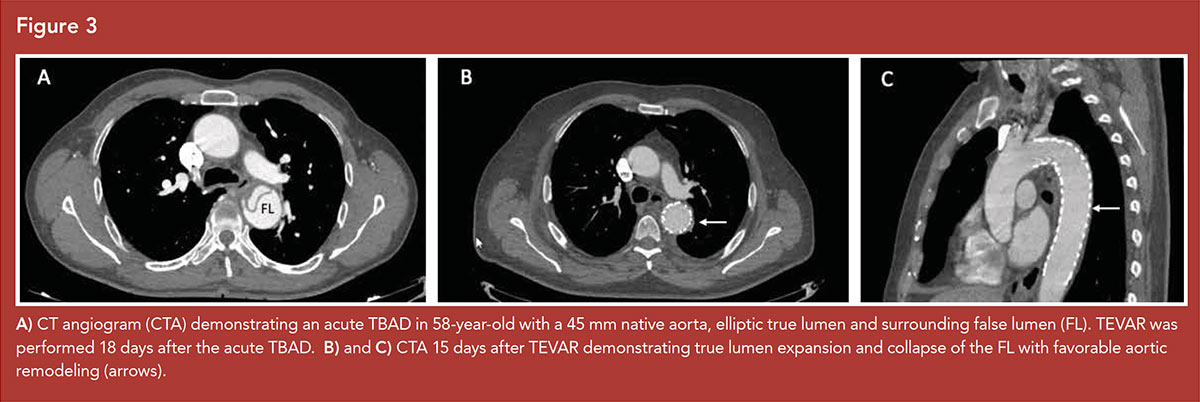
Imaging features are predictive of outcomes in uncomplicated TBAD and associate with increased risk of aortic growth and mid-term and late aneurysm formation5 (Figures 2, 3).
A total aortic diameter of >40 mm in the acute phase predicts aortic growth in several studies, while a diameter of <40 mm was a negative predictor.22,27,28
Focusing on the aortic diameter, a distinction between the total aortic diameter and the FL diameter should be made. A FL diameter of >22 mm in the upper descending thoracic aorta on initial imaging was an independent predictor for aortic enlargement during follow-up.27
Multiple radiologic predictors, including patent FL, partial thrombosis of the FL, FL diameter >22 mm, elliptic TL combined with round FL, single entry tear, and entry tear size >10 mm, may all be interrelated due to pressurization of the FL, with subsequent aortic enlargement of the dissected segment (Figure 2).24,27,28
The site of the proximal entry tear should also be considered as a predictor for pre-emptive TEVAR. In a recent paper, surgeons were asked to rank anatomical factors in order of importance before TEVAR in uncomplicated TBAD.32
Primary entry tear location on concavity/inner curve of thoracic aorta and proximal sealing zone length <20 mm requiring multiple vessel debranching were considered primary risk factors to avoid TEVAR in uncomplicated TBAD.32
"Hic sunt leones" ("here are lions") Romans used to write on maps, meaning that a dangerous or unknown situation could be waiting for them in uncharted lands. The same attitude should be considered when facing TEVAR in uncomplicated TBAD.
Correct timing, age and gender selection, underlying aortopathy, long-term prognosis, anatomical evaluation and surgeon experience are important issues to consider when contemplating TEVAR in initially uncomplicated TBAD patients.
Surgical Therapy For Acute Complicated TBAD
Since its inception in the early 1950s, surgical replacement of acute TBAD was the standard of care until the early 1970s.33 Conceptualization of dp/dt by Wheat and colleagues and use of anti-impulse therapy (beta-blocker) and tight blood pressure control led to a change in the treatment paradigm in TBAD to primary medical therapy with significant improvement in outcomes.34
Subsequently, the surgical replacement of the descending thoracic aorta in TBAD was reserved for failure of medical therapy, ruptures and malperfusion syndromes.35 In the current era of endografts, open repair for complicated TBAD is reserved for patients who are not anatomic candidates for TEVAR. In patients with TBAD and malperfusion or failed medical therapy, this would include any patient with proximal landing zone >42 mm. In patients with TBAD and rupture, this would be a proximal or distal landing zone >42 mm.36
Individuals with heritable thoracic aortic disease (Marfan Syndrome, Loeys-Dietz syndrome, etc.) require shared-decision making. While endovascular therapy may be lifesaving in complicated TBAD, adverse outcomes may be more common and re-interventions more frequent.31,37
While clamp and sew was the predominant technique in early years, this was replaced by distal aortic perfusion, and later total cardiopulmonary bypass with circulatory arrest.38 The latter two techniques still have the best outcomes regarding spinal cord ischemia, stroke and renal failure compared to clamp and sew, especially when combined with administration of steroids, measurement of somatosensory evoked potentials and evaluation of motor-evoked potentials. The advent of spinal cord drainage further reduced the risk of immediate or delayed paraparesis and paraplegia.39 All these advances, along with an improvement in postoperative care, have resulted in a significant reduction in morbidity and mortality.
Patients undergoing replacement of the descending thoracic aorta for complicated TBAD have substantially higher operative mortality (~25%) compared to cohorts with aneurysm or chronic dissection.40-43
Endovascular Therapy in Type A Aortic Dissection
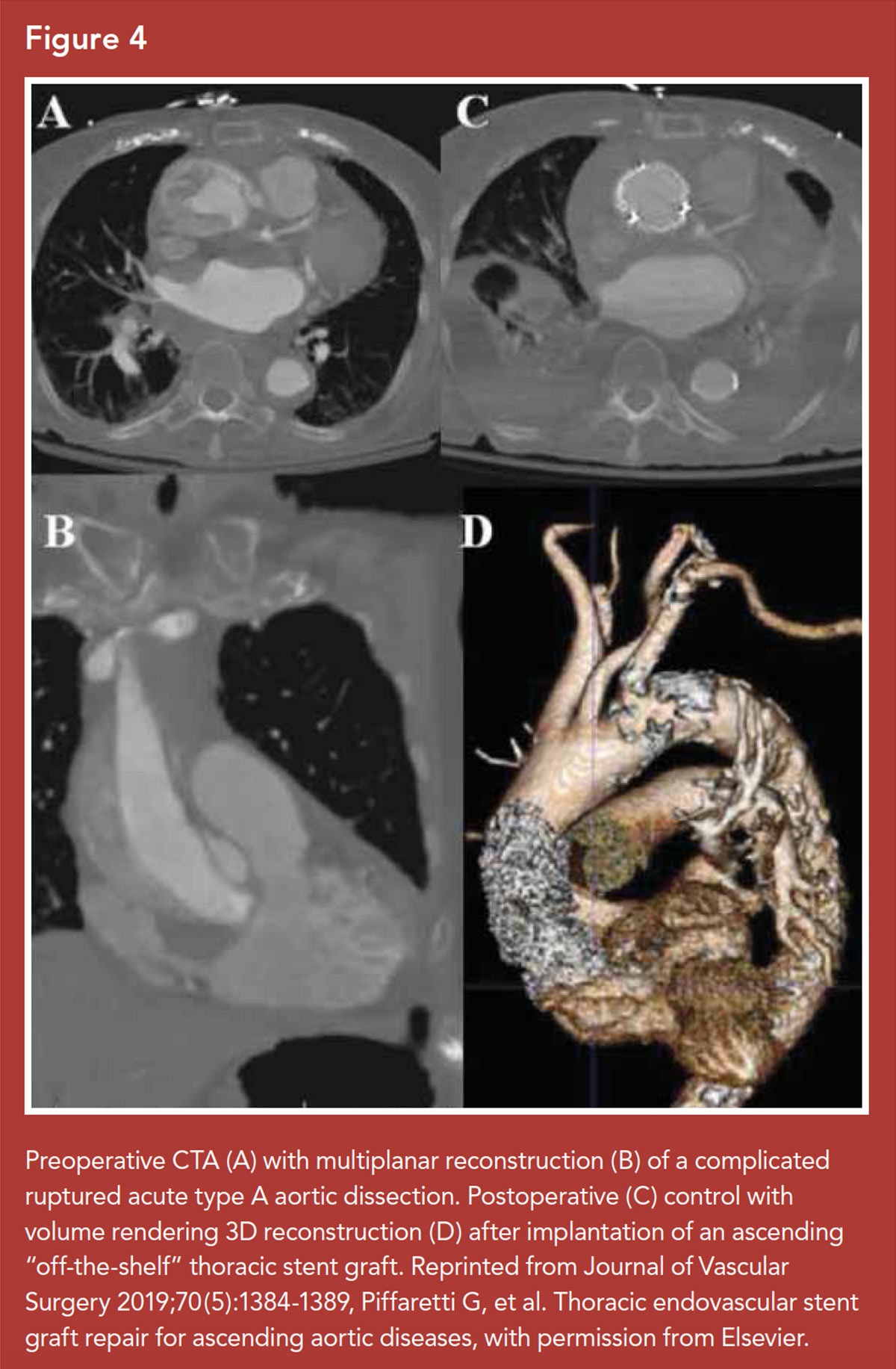
Endovascular therapy for TAAD has been proposed as an alternative to open repair for selected patients (Figure 4).44 The anatomical characteristics of the ascending aorta were once considered an insurmountable challenge for stent grafting.
However, ascending thoracic endovascular aortic repair (aTEVAR) has proven viable in very selective cases of TAAD (acute or chronic) considered unfit for conventional operations, or in patients with previous cardiac surgery.
The ascending aorta is a relatively short, curved artery, interposed between the aortic valve and the origin of coronary arteries proximally and the supra-aortic branches distally, leaving minimal length for suitable landing zones.
Successful stent graft deployment must also contend with not only the differential lengths of the inner and outer curves, but also the high shear force and wall tension generated by the close proximity to the left ventricle.
In addition to these anatomical challenges, a number of important clinical characteristic preclude aTEVAR:
- presence of a mechanical valve,
- severe aortic valve regurgitation,
- genetic aortopathy such as Marfan or Loeys-Dietz syndrome,
- patent coronary artery bypass grafts originating from the mid-ascending aorta,
- severe aortic root disease.
Additionally, isolated ascending aortic pathology is relatively uncommon, as the majority of TAAD cases involve multiple aortic segments such as the aortic arch and/or the descending aorta, favoring open repair or a complex hybrid operation, rather than a single-staged aTEVAR.
Despite these anatomical and technical challenges, a few purpose-built devices have been designed and used specifically for ascending aortic endovascular repair, introducible by transfemoral or transapical approaches.45 A recent systematic review of 92 patients demonstrated an overall technical success rate of 96% and a 30-day mortality of 9%.
Stroke and early endoleak rates were 6% and 18%, respectively, with a re-intervention rate of 15%.46 Although the authors reported encouraging in-hospital and short-term results, further data are required to demonstrate mid- and long-term effectiveness.
Additional technical and clinical research, supported by experts in endovascular repair and transcatheter valve replacement, is needed to provide clearer indication criteria for aTEVAR.
Ongoing engineering research and design are required to address the complexity of the aortic valve, aortic root and heterogeneous ascending aortic morphology, as well as the impact of increased hemodynamic stress in the proximal aorta when contemplating endovascular approaches to ascending dissection.47-49
Until then, aTEVAR for TAAD will continue to play a secondary role, with urgent open surgical repair the gold standard treatment.
Conclusions
Due to technological and procedural advances, interventional therapy has become the preferred method of treating acute complications of Type B aortic dissection leading to lower morbidity and mortality compared to emergency open surgery. Procedural risks, re-interventions and anatomic limitations remain, but innovations in endovascular therapy will continue to improve this very important treatment for aortic dissection.







This article was authored by Alan C. Braverman, MD, FACC, Cardiovascular Division, Washington University School of Medicine, St. Louis, MO; Santi Trimarchi, MD, PhD, Vascular Surgery, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico Milan, University of Milan, Italy; Ibrahim Sultan, MD, FACC, Department of Cardiothoracic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA; George Arnaoutakis, MD, Division of Thoracic and Cardiovascular Surgery, University of Florida, Gainesville; Valerio Stefano Tolva, MD, PhD, Department of Vascular Surgery, ASST Grande Ospedale Metropolitano Niguarda. Milano. Italy; Ali Khoynezhad, MD, PhD, FACC, Department of Cardiovascular Surgery, MemorialCare Heart and Vascular Institute, Long Beach, CA; and Mark D. Peterson, MD, PhD, Division of Cardiac Surgery, University of Toronto, Canada.
References
1. Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the international registry of acute aortic dissection: A 20-year experience of collaborative clinical research. Circulation 2018;137:1846-60.
2. Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type b aortic dissections. Ann Thorac Surg 2020;109:959-81.
3. Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation 2014;130(11 Suppl 1):S45-50.
4. Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet 2015;385:800-11.
5. Tadros RO, Tang GHL, Barnes HJ, et al. Optimal treatment of uncomplicated type b aortic dissection: JACC review topic of the week. J Am Coll Cardiol 2019;74:1494-1504.
6. Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J 2006;27;489-98.
7. Riambau V, Bockler D, Brunkwall J, et al. Editor's choice - management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017;53:4-52.
8. Reutersberg B, Trenner M, Haller B. et al. The incidence of delayed complications in acute type B aortic dissections is underestimated. J Vasc Surg 2018;68:356-63.
9. Trimarchi S, Segreti S, Grassi V, et al. Emergent treatment of aortic rupture in acute type B dissection. Ann Cardiothorac Surg 2014;3:319-24.
10. Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16.
11. Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: A report from the international registry of acute aortic dissection (IRAD). JACC Cardiovasc Interv 2013;6:876-82.
12. Fattori R, Cao P, De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol 2013;61:1661-78.
13. Kilic A, Sultan IS, Arnaoutakis GJ, et al. Assessment of thoracic endografting operative mortality risk score: Development and validation in 2,000 patients. Ann Thorac Surg 2015;100:860-67.
8. Cambria RP, Crawford RS, Cho JS, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg 2009;50:1255-64; e1251-54.
9. Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: Is surgery still the best option? A report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1:395-402.
10. Lou X, Duwayri YM, Jordan WD, Jr., et al. The safety and efficacy of extended TEVAR in acute type B aortic dissection. Ann Thorac Surg 2020;110:799-806.
11. Xue Y, Ge Y, Ge X, et al. Association between extent of stent-graft coverage and thoracic aortic remodeling after endovascular repair of type b aortic dissection. J Endovasc Ther 2020;27:211-22.
12. Bianco V, Sultan I, Kilic A, et al. Concomitant left subclavian artery revascularization with carotid-subclavian transposition during zone 2 thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2020;159:1222-27.
13. Ma T, Dong ZH, Fu WG, et al. Incidence and risk factors for retrograde type a dissection and stent graft-induced new entry after thoracic endovascular aortic repair. J Vasc Surg 2018;67:1026-33.
14. Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg 2014;260:389-95.
15. Chen Y, Zhang S, Liu L, et al. Retrograde type a aortic dissection after thoracic endovascular aortic repair: A systematic review and meta-analysis. J Am Heart Assoc 2017;22;6:e004649.
16. Sultan I, Dufendach K, Kilic A et al. Bare metal stent use in type B aortic dissection may offer positive remodeling for the distal aorta. Ann Thorac Surg 2018;106:1364-70.
17. Pruitt EY, Scali ST, Arnaoutakis DJ, et al. Complicated acute type B aortic dissection: update on management and results. J Cardiovasc Surg (Torino) 2020;61:697-707.
18. Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients with type B acute aortic dissection: insight from the International Registry of Acute Aortic Dissection. Circulation 2006; 114:2226-31.
19. Umaña JP, Lai DT, Mitchell RS, et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J Thorac Cardiovasc Surg 2002; 124:896-910.
20. Schwartz SI, Durham C, Clouse WD, et al. Predictors of late aortic intervention in patients with medically treated type B aortic dissection. J Vasc Surg 2018;67:78-84.
21. Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-28.
22. Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized Investigation of Stent Grafts in Aortic Dissection trial. Circ Cardiovasc Interven 2013;6:407-16.
23. Kamman AV, Brunkwall J, Verhoeven EL, Heijmen RH, Trimarchi S; ADSORB trialists. Predictors of aortic growth in uncomplicated type B aortic dissection from the Acute Dissection Stent Grafting or Best Medical Treatment (ADSORB) database. J Vasc Surg 2017;65:964-71.
24. Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50:799-804.
25. Desai ND, Gottret JP, Szeto WY, et al. Impact of timing on major complications after thoracic endovascular aortic repair for acute type B aortic dissection. J Thorac Cardiovasc Surg 2015;149(2 Suppl):S151-6.
26. VIRTUE Registry Investigators. Mid-term outcomes and aortic remodelling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg 2014;48:363-71.
27. Trimarchi S, Jonker FH, van Bogerijen GH, et al. Predicting aortic enlargement in type B aortic dissection. Ann Cardiothorac Surg 2014;3:285-91.
28. Jonker FH, Trimarchi S, Rampoldi V, et al. International Registry of acute aortic dissection (IRAD) investigators. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg 2012;94:1223-9.
29. Chaddha A, Erickson S, Kline-Rogers E, et al. Medication adherence patterns in aortic dissection survivors. Indian J Med Res 2018;147:183-88.
30. Dong J, Duan X, Feng R, et al. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci Rep 2017;7:43957.
31. Eid-Lidt G, Gaspar J, Meléndez-Ramírez G, et al. Endovascular treatment of type B dissection in patients with Marfan syndrome: mid-term outcomes and aortic remodeling. Catheter Cardiovasc Interv 2013;82:E898-905.
32. Munshi B, Doyle BJ, Ritter JC, et al. Surgical decision making in uncomplicated Type B aortic dissection: A survey of Australian/New Zealand and European surgeons. Eur J Vasc Endovasc Surg 2020;60:194-200.
33. Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995;92(9 Suppl):II113-21.
34. Prokop EK, Palmer RF, Wheat MW Jr. Hydrodynamic forces in dissecting aneurysms. In-vitro studies in a Tygon model and in dog aortas. Circ Res 1970;27:121-7.
35. Khoynezhad A, Gupta PK, Donayre CE, White RA. Current status of endovascular management of complicated acute type B aortic dissection. Future Cardiol 2009;5:581-88.
36. Khoynezhad A, Donayre CE, Smith J, et al. Risk factors for early and late mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2008;135:1103-9, 1109.e1-4.
37. Pellenc Q, Girault A, Rousse A, et al. Optimising aortic endovascular repair in patients with marfan syndrome. Eur J Vasc Endovasc Surg 2020;59:577-85.
38. Khoynezhad A, Bello R, Smego DR, et al. Improved outcome after repair of descending and thoracoabdominal aortic aneurysms using modern adjuncts. Interact Cardiovasc Thorac Surg 2005;4:574-76.
39. Safi HJ, Campbell MP, Miller CC 3rd, et al. Cerebral spinal fluid drainage and distal aortic perfusion decrease the incidence of neurological deficit: the results of 343 descending and thoracoabdominal aortic aneurysm repairs. Eur J Vasc Endovasc Surg 1997;14:118-24.
40. Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg 2008;85:965-70.
41. Trimarchi S, Nienaber CA, Rampoldi V, et al. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114(1 Suppl):I357-64.
42. Estrera AL, Miller CC 3rd, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg 2005;80:1290-6.
43. Minatoya K, Ogino H, Matsuda H, et al. Replacement of the descending aorta: recent outcomes of open surgery performed with partial cardiopulmonary bypass. J Thorac Cardiovasc Surg 2008;136:431-35.
44. Trimarchi S, Grassi V, Lomazzi C, et al. Endovascular type A aortic repair-When? J Card Surg 2020;Oct 8:[Epub ahead of print].
45. Shah A, Khoynezhad A. Thoracic endovascular repair for acute type A aortic dissection: operative technique. Ann Cardiothorac Surg 2016;5:389-96.
46. Ahmed Y, Houben IB, Figueroa CA, et al. Endovascular ascending aortic repair in type A dissection: A systematic review. J Card Surg 2020;Nov 10:[Epub ahead of print].
47. Tobey DJ, Reynolds TS, Kopchok GE, et al. In vivo assessment of ascending and arch aortic compliance. Ann Vasc Surg 2019;57:22-28.
48. Nauta FJ, Conti M, Marconi S, et al. An experimental investigation of the impact of thoracic endovascular aortic repair on longitudinal strain. Eur J Cardiothorac Surg 2016;50:955-61.
49. Nauta FJH, de Beaufort HWL, Conti M, et al. Impact of thoracic endovascular aortic repair on radial strain in an ex vivo porcine model. Eur J Cardiothorac Surg 2017;51:783-9.
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Vascular Medicine, Aortic Surgery, Cardiac Surgery and CHD and Pediatrics, Congenital Heart Disease, CHD and Pediatrics and Imaging, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Interventions and Imaging, Interventions and Structural Heart Disease, Interventions and Vascular Medicine, Angiography, Computed Tomography, Echocardiography/Ultrasound, Nuclear Imaging
Keywords: ACC Publications, Cardiology Magazine, Aneurysm, Dissecting, Angiography, Angiotensin-Converting Enzyme Inhibitors, Aortic Aneurysm, Thoracic, Aorta, Thoracic, Aorta, Aorta, Aortic Diseases, Aortic Rupture, Aortic Valve, Blood Vessel Prosthesis Implantation, Blood Pressure, Calcium Channel Blockers, Cardiac Surgical Procedures, Cardiopulmonary Bypass, Carotid Intima-Media Thickness, Carotid Artery, Common, Concept Formation, Celiac Artery, Coronary Artery Bypass, Decision Making, Dissection, Dilatation, Coronary Vessels, Endoleak, Drainage, Endovascular Procedures, Evoked Potentials, Somatosensory, Fibrin Fibrinogen Degradation Products, Follow-Up Studies, Heart Rate, Heart Ventricles, Evoked Potentials, Motor, Hemodynamics, Imaging, Three-Dimensional, Imaging, Three-Dimensional, Ischemia, Hospitals, Hospital Mortality, Laboratories, Hematoma, Loeys-Dietz Syndrome, Lower Extremity, Medicine, Marfan Syndrome, Life Style, Pain, Intractable, Paraparesis, Morbidity, Paraplegia, Pleural Effusion, Perfusion, Pulmonary Disease, Chronic Obstructive, Postoperative Care, Random Allocation, Reference Standards, Prognosis, Risk Assessment, Risk Factors, Renal Insufficiency, Smoking Cessation, Spinal Cord Ischemia, Stents, Sternotomy, Steroids, Standard of Care, Registries, Stroke, Subclavian Artery, Surgeons, Syndrome, Thrombosis, Tomography, Tomography, X-Ray Computed, Treatment Outcome, Triticum, Universities, Virtues
< Back to Listings