Focus on EP | Single-shot Techniques For Pulmonary Vein Isolation in AFib: From Cryoablation to Pulse Field Ablation
Radiofrequency (RF) and cryothermy remain the two dominant forms of energy delivered during catheter-based pulmonary vein isolation (PVI) for atrial fibrillation (AFib) ablation. First described for the treatment of focal AFib triggers in 1998,1 RF ablation is now used to achieve PVI with continuous, point-by-point application of thermal injury around the antrum of each PV (or PV pair). However, this approach has been viewed by many electrophysiologists as time-consuming, high-risk for procedural complications, and suboptimal for achieving durable PVI.
Cryoablation balloon therapy, introduced over a decade later, developed out of a desire for a faster ablation strategy that could provide safer and more durable PVI. With the modern day cryoablation approach, PVI can often be achieved with a single circumferential ablation lesion. Ablation technologies that offer an expedited alternative to the traditional point-by-point PVI approach have become known as single-shot techniques (Figure 1).
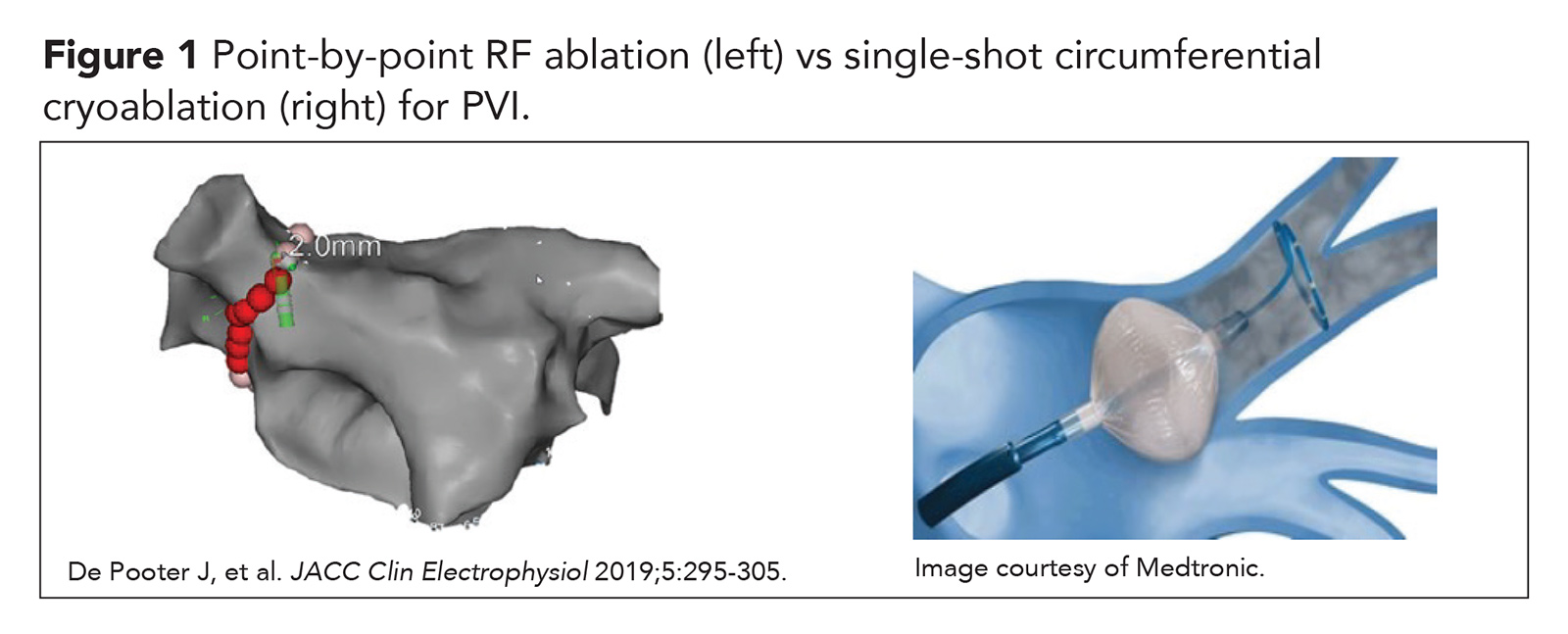
Cryoablation in Drug-Refractory Symptomatic Paroxysmal AFib
First presented as a late-breaking study at Heart Rhythm in 2010 and later published in 2013, STOP AF was the first large randomized clinical trial to assess the safety and efficacy of cryoablation in patients with symptomatic paroxysmal AFib in whom antiarrhythmic drug (AAD) therapy had failed.2,3 Of the patients randomized to cryoballoon ablation (n=163), acute PVI was achieved in 97.6% and acute, serious procedure-related events occurred in 3.1%. At 12 months, the primary combined effectiveness endpoint (freedom from AFib after blanking period, avoidance of additional AADs and avoidance of additional AFib-related intervention) was seen in 69.9% and 7.3% of the cryoablation and AAD groups, respectively (p<0.001).
Notable ablation-related adverse events included phrenic nerve paralysis (11.2%), PV stenosis (3.1%) and stroke (2.2%). No atrial-esophageal fistulas were seen. Overall serious adverse event rates over 12 months were not different in the cryoablation and AAD groups (12.3% vs. 14.6%; p=0.688).
Based on these results, the U.S. Food and Drug Association (FDA) approved the first cryoballoon ablation system on December 12, 2010 (Arctic Front Cardiac CryoAblation Catheter System, Medtronic).4 Successive iterations of this cryoballoon ablation system have also received FDA approval, including the second-generation Arctic Front Advance catheter in 2012 and the current third-generation Arctic Front Advance Pro catheter in 2018.5
Cryoablation vs. RF Ablation in Drug-Refractory Symptomatic Paroxysmal AFib
Subsequent head-to-head comparison of RF and cryoballoon ablation techniques in two randomized controlled trials have demonstrated comparable safety and efficacy outcomes between the two approaches. In the 2016 FIRE and ICE study, symptomatic patients with drug-refractory paroxysmal AFib were randomized to undergo PVI with either RF ablation (n=384) or cryoballoon ablation (n=378).6 Of note, the majority of ablation in the RF group was achieved without a contact force-sensing catheter (76%) and the majority of ablation in the cryoballoon group was achieved with the second-generation catheter (76%).
After a mean duration of 1.5 years follow-up, the primary efficacy endpoint (recurrence of atrial tachyarrhythmia after blanking period, avoidance of AADs and avoidance of repeat ablation) occurred in 34.6% of patients in the cryoballoon group and 35.9% of the RF group (1-year Kaplan-Meier event-rate estimates, p<0.001 for noninferiority).
The primary safety endpoint in FIRE and ICE (all-cause death, stroke or transient ischemic stroke, and serious adverse events) occurred in 10.2% and 12.8% of the cryoballoon and RF groups (1-year Kaplan-Meier event-rate estimates, p=0.24). The mean total procedure time was significantly shorter with cryoablation vs. RF (124 vs. 141 minutes; p<0.001) but mean total fluoroscopy time was significantly shorter with RF vs. cryoablation (17 vs. 22 minutes; p<0.001).
In the more recent 2019 CIRCA-DOSE study, similar results were seen in symptomatic patients with drug-refractory paroxysmal AFib randomized to undergo PVI with RF ablation using a contact force-guided catheter (n=115) or cryoballoon ablation using the second-generation catheter for either a 2-minute lesion (n=116) or a 4-minute lesion (n=115).7 All patients received an implantable loop recorder.
At one year, there was no significant difference in the primary effectiveness endpoint (freedom from atrial tachyarrhythmia after blanking period) between the RF group, 2-minute cryoablation group and 4-minute cryoablation group (53.9% vs. 51.7% vs. 52.2%; p=0.59). Similarly, there was no significant difference in serious adverse events across the RF group, 2-minute cryoablation group and 4-minute cryoablation group (2.6% vs. 6.0% vs. 5.2%; p=0.31). Phrenic nerve injury was observed in three patients who underwent cryoablation (1.3%), esophageal injury in one patient who underwent RF ablation (<1%), and no pulmonary vein stenosis or procedure-related deaths occurred.
Notably, in CIRCA-DOSE, the mean procedure time was again significantly shorter in the cryoablation groups vs. the RF group (130.5 minutes in 2-minute cryoablation group vs. 164.5 minutes in RF group; p<0.001; and 143.0 minutes in 4-minute cryoablation group, p<0.001) but the mean total fluoroscopy time was again significantly shorter in the RF ablation group vs. either cryoballoon groups (19.0 minutes in the 2-minute cryoablation group vs. 5.2 minutes in RF group; p<0.001; and 17.2 minutes in 4-minute cryoablation group; p<0.001).
Cryoablation Therapy as First-Line For Symptomatic Paroxysmal AFib
Perhaps the most impressive outcomes for cryoablation guided-PVI were recently demonstrated in the STOP AF First and EARLY-AF trials of 2021, which not only revealed superiority of cryoablation therapy over AAD as first-line therapy for preventing atrial tachyarrhythmia recurrence in patients with symptomatic paroxysmal AFib, but also confirmed the low procedural risk associated with cryoablation therapy. In the STOP AF First study, patients with symptomatic paroxysmal AFib were randomized to either initial treatment with cryoablation (n=104) or with AADs (n=99).8
At 12-months, the primary combined efficacy endpoint (freedom from initial procedure failure or atrial arrhythmia recurrence after blanking period) was achieved in 74.6% and 45.0% of patients in the ablation and AAD groups (p<0.001).
The primary combined safety endpoint (related to procedural complications and evaluated only in the ablation group) occurred in 1.9% of patients and met the prespecified performance threshold of <12% (p<0.001).
In the EARLY-AF study, patients with symptomatic paroxysmal AFib were randomized to either initial treatment with cryoablation (n=144) or with AADs (n=149).9 All patients received an implantable loop recorder. At 12 months, the primary combined efficacy endpoint (freedom from atrial arrhythmia recurrence after blanking period or avoidance of AAD therapy) was achieved in 57.1% of patients in the ablation group and 32.2% of patients in the AAD group (p<0.001). Serious adverse events occurred in 3.2% and 4.0% of patients in the ablation and AAD groups (P=NS).
In the race between ablation strategies to demonstrate the most favorable safety and efficacy profile in the initial treatment of paroxysmal AFib, cryoablation therapy appears to be the winner.
A meta-analysis of the randomized controlled trials evaluating RF ablation against AAD as first-line therapy also had demonstrated a significantly improved efficacy outcome with RF ablation but at the cost of significantly increased serious adverse events.10
The Rise of Pulse Field Ablation
Over the past decade, the success of cryoballoon ablation therapy has spurred extensive innovation to develop the next single-shot technology that would provide an even faster, safer and more effective approach to PVI. Novel single-shot ablation systems employing RF, laser and ultrasound energy have been explored, but none have gained significant traction as a PVI strategy. This ongoing search for the next game-changing ablation eventually culminated in the late-breaking trial announcement of the first-in-human report of pulse field ablation (PFA) at the 2018 Heart Rhythm Society Scientific Sessions.11
Unlike RF and cryothermy ablation, which utilize different ends of the thermal energy spectrum (extreme heat or extreme cold) to create nonselective tissue injury, PFA delivers electrical energy to create tissue-selective injury in a process called electroporation. During catheter-based PFA, a localized electrical field disturbance is created at the distal catheter using ultra-rapid, high-voltage electrical pulses. This electrical field results in pore formation in the cell membrane (electroporation), which leads to unregulated movement of molecules and ions into and out of the cell. When an electrical field of sufficient strength is applied, irreversible electroporation occurs resulting in cell death. Unlike current ablation modalities, PFA can be modulated to selectively target myocardial tissue while avoiding injury to nearby structures (particularly the esophagus and phrenic nerve; Figure 2).
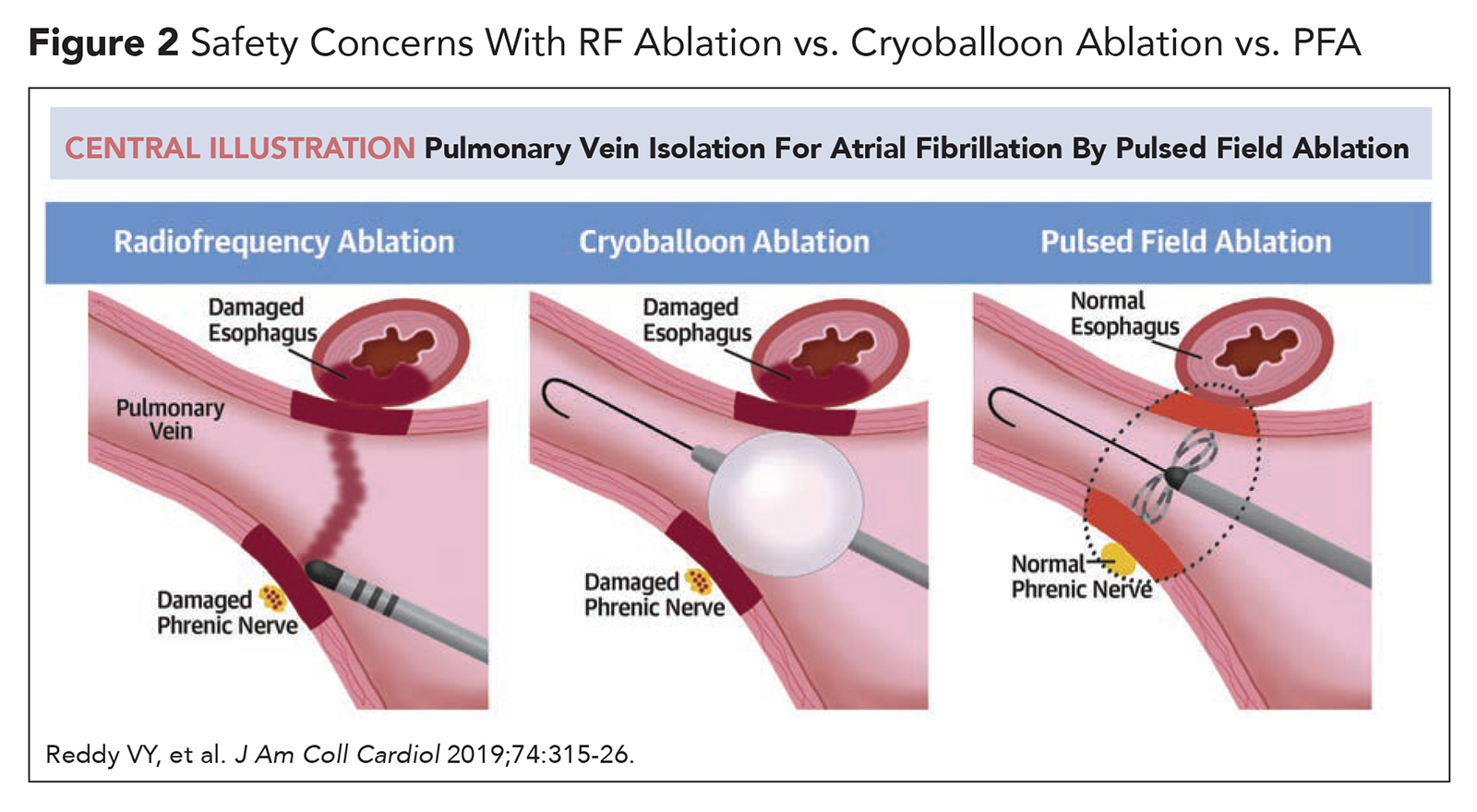
Furthermore, PVI with PFA is not as reliant on catheter force or stability: effective lesions can be achieved as long as the ablation catheter is seated in the PV antrum during the brief delivery of electrical pulses.12
Three PFA systems are currently undergoing clinical evaluation, each incorporating a multielectrode ablation catheter designed to engage the PVs and a pulse generator that delivers a proprietary high-voltage, high-frequency electrical waveform. The Medtronic and Biosense PFA catheters, the PVAC GOLD and VARIPULSE, respectively, are lasso-shaped while the Farapulse PFA catheter, the FARAWAVE, can be deployed in either a basket or flower petal configuration. All PFA systems utilize a similar procedural workflow to current catheter ablation setups, including femoral venous groin access, transseptal puncture, and pairing with an electroanatomical mapping system.13-15
First-in-man feasibility studies on PFA outcomes in 121 patients with paroxysmal AFib (IMPULSE, PEFCAT and PEFCAT II) have shown promising results.16 Of note, in these studies, PFA was delivered with a pulse waveform that was optimized throughout the study period, and only the final 49 patients in the cohort were treated with an optimized biphasic PFA waveform (PFA-OW). Acute complete PVI was achieved in 100% of patients who underwent PFA with a mean procedure time of 96.2 minutes and a mean fluoroscopy time of 13.7 minutes.
In the subset of patients (n=121) who underwent PV remapping at a mean of 93.0 +/- 30.1 days postprocedure, durable PVI was observed in 84.8% of PVs and 64.5% of patients. In the PFA-OW group (n=44), durable PVI was observed in 96% of PVs and 84.1% of patients.
At one-year follow-up, freedom from atrial arrhythmia recurrence after a single procedure was 79.3% in the total cohort and 84.5% in the PFA-OW group. Serious adverse events occurred in four patients (3.3%): two patients developed pericardial effusion requiring pericardiocentesis, one patient developed a vascular hematoma and one patient had a transient ischemic attack. Impressively, there were no instances of esophageal damage, PV stenosis, phrenic nerve injury, stroke or death.
Ongoing clinical trials investigating the safety and efficacy of PFA include the ADVENT study (randomized study of PFA vs. RF and cryoablation in patients with paroxysmal AF, using the Farapulse PFA system),17 PULSED AF study (observational study of PFA in patients with paroxysmal and early-persistent AFib, using the Medtronic PFA system),18 and the INSPIRE study (observational study of PFA in patients with paroxysmal AFib, using the Biosense PFA system).19
Like all novel medical technologies, PFA will need to undergo comprehensive clinical evaluation before it will be considered for FDA approval. However, barring any major safety issues, PFA will likely be available for routine clinical use within the next several years upon completion of the aforementioned clinical trials. Not only does PFA appear to enhance the safety and efficacy of durable PVI, but it will also decrease the length of the procedure, obviate the need for general anesthesia and urinary catheter placement, and potentially allow for same-day discharge. All things considered, PFA may become the ultimate single-shot technique for PVI.

This article was authored by Edward Chu, MD, (@Ed_Chu_ MD), electrophysiology Fellow in Training (FIT) at Mount Sinai Medical Center in New York.
References
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66.
- Noonan P, Hills MT. STOP AF Cryoablation for Atrial Fibrillation: Quality of Life Results and Technical Considerations. Available here. Accessed May 16, 2021.
- Packer DL, Kowal RC, Wheelan KR, et al; STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713-23.
- Press Release. Medtronic Receives FDA Approval for First and Only Cryoballoon Ablation Treatment in the U.S. for Paroxysmal Atrial Fibrillation. Available here. Accessed May 16, 2021.
- U.S Food & Drug Administration Premarket Approval (PMA). Available here. Accessed May 16, 2021.
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235-45.
- Andrade JG, Champagne J, Dubuc M, et al; CIRCA-DOSE Study Investigators. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779-88.
- Wazni OM, Dandamudi G, Sood N, et al; STOP AF First Trial Investigators. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316-24.
- Andrade JG, Wells GA, Deyell MW, et al; EARLY-AF Investigators. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305-15.
- Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 2015;17:370-8.
- First Human Cases Treated With Globe Mult-electrode Contact Mapping and Ablation System. Heart Rhythm 2018. Available here. Accessed May 16, 2021.
- Ramirez FD, Reddy VY, Viswanathan R, et al. Emerging technologies for pulmonary vein isolation. Circ Res 2020;127:170-83.
- Stewart MT, Haines DE, Verma A, et al. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm 2019;16:754-64.
- Yavin H, Brem E, Zilberman I, et al. Circular multielectrode pulsed field ablation catheter lasso pulsed field ablation: lesion characteristics, durability, and effect on neighboring structures. Circ Arrhythm Electrophysiol 2021;14:e009229.
- Farapulse. Available here. Accessed May 16, 2021.
- Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614-27.
- ClinicalTrials.gov. The Farapulse Advent Pivotal Trial (ADVENT). Available here. Accessed May 16, 2021.
- ClinicalTrials.gov. Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF (PULSED AF). Available here. Accessed May 16, 2021.
- ClinicalTrials.gov. A Study for Treatment of Paroxysmal Atrial Fibrillation (PAF) by Pulsed Field Ablation (PFA) System With Irreversible Electroporation (IRE) (inspIRE). Available here. Accessed May 16, 2021.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Pericardial Disease, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Interventions and Imaging, Nuclear Imaging
Keywords: ACC Publications, Cardiology Magazine, Anesthesia, General, Anti-Arrhythmia Agents, Atrial Fibrillation, Brain Ischemia, Catheter Ablation, Cell Death, Cardiac Catheters, Cell Membrane, Cryosurgery, Constriction, Pathologic, Electroporation, Esophageal Fistula, Esophagus, Family Characteristics, Feasibility Studies, Fluoroscopy, Follow-Up Studies, Hematoma, Ischemic Attack, Transient, Paralysis, Paralysis, Patient Discharge, Pericardiocentesis, Pharmaceutical Preparations, Phrenic Nerve, Pulmonary Veins, Tachycardia, Stroke, Technology, Urinary Catheters, Workflow, Traction
< Back to Listings



